How Many Neutrons Does Silver Have
Kalali
Apr 07, 2025 · 5 min read
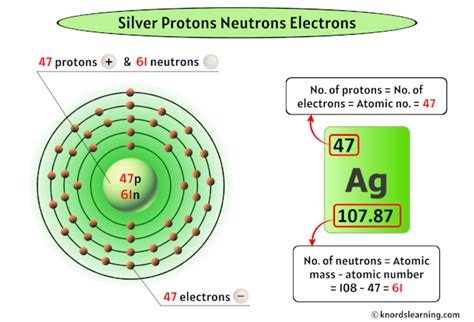
Table of Contents
How Many Neutrons Does Silver Have? Unveiling the Isotopic Complexity of a Precious Metal
Silver, a lustrous transition metal prized for its conductivity and beauty, presents a fascinating case study in nuclear physics due to its isotopic complexity. Unlike elements with a single dominant isotope, silver boasts two naturally occurring isotopes, each with a different number of neutrons. Understanding the neutron count in silver requires delving into the intricacies of isotopes and their prevalence in nature. This article will explore the isotopic composition of silver, explaining the number of neutrons in each isotope and the implications of this variation.
Understanding Isotopes: The Building Blocks of Elements
Before we dive into the specifics of silver's neutron count, let's briefly review the concept of isotopes. Atoms of the same element, defined by their atomic number (number of protons), can possess varying numbers of neutrons. These variations are called isotopes. While the number of protons dictates the element's chemical properties, the number of neutrons influences its nuclear stability and mass. Isotopes of the same element exhibit similar chemical behavior but can differ significantly in physical properties, such as mass and radioactivity.
The Role of Neutrons in Atomic Stability
Neutrons play a crucial role in maintaining the stability of an atom's nucleus. The strong nuclear force, which binds protons and neutrons together, is responsible for holding the nucleus intact. However, the electrostatic repulsion between positively charged protons can destabilize the nucleus, particularly in heavier atoms. Neutrons, lacking a charge, help to counterbalance this repulsive force, contributing to nuclear stability. An imbalance in the proton-neutron ratio can lead to radioactive decay, where the unstable nucleus emits particles to achieve a more stable configuration.
Silver's Isotopic Composition: A Closer Look at ¹⁰⁷Ag and ¹⁰⁹Ag
Silver (Ag) has an atomic number of 47, meaning every silver atom possesses 47 protons. However, the number of neutrons varies, leading to the existence of two primary isotopes: ¹⁰⁷Ag and ¹⁰⁹Ag.
¹⁰⁷Ag: The Lighter Isotope
¹⁰⁷Ag, representing approximately 51.84% of naturally occurring silver, has a mass number of 107. This mass number represents the total number of protons and neutrons in the nucleus. Since it has 47 protons, ¹⁰⁷Ag contains 107 - 47 = 60 neutrons.
Key characteristics of ¹⁰⁷Ag:
- Mass number: 107
- Number of protons: 47
- Number of neutrons: 60
- Natural abundance: ~51.84%
- Stable isotope: Does not undergo radioactive decay.
¹⁰⁹Ag: The Heavier Isotope
¹⁰⁹Ag accounts for roughly 48.16% of naturally occurring silver. Its mass number is 109, which, given 47 protons, implies 109 - 47 = 62 neutrons.
Key characteristics of ¹⁰⁹Ag:
- Mass number: 109
- Number of protons: 47
- Number of neutrons: 62
- Natural abundance: ~48.16%
- Stable isotope: Does not undergo radioactive decay.
The Significance of Silver's Isotopic Abundance
The nearly equal abundance of ¹⁰⁷Ag and ¹⁰⁹Ag is a noteworthy feature of silver's isotopic composition. This even distribution contrasts with many other elements where one isotope significantly dominates. The nearly 50/50 split influences the average atomic mass of silver, which is approximately 107.87 amu (atomic mass units), reflecting the contribution of both isotopes.
This relatively balanced isotopic distribution has implications in various scientific and technological applications. For instance, in analytical techniques such as mass spectrometry, the presence of two isotopes needs to be considered for accurate measurements and interpretations. The different masses of the isotopes can also subtly influence the physical properties of silver materials, although these effects are generally minor.
Beyond the Two Stable Isotopes: Exploring Radioactive Silver Isotopes
While ¹⁰⁷Ag and ¹⁰⁹Ag are the most common and stable silver isotopes, several other radioactive isotopes of silver have been synthesized in laboratories. These radioactive isotopes have varying numbers of neutrons and exhibit different decay modes, such as beta decay or electron capture. These isotopes have short half-lives, meaning they decay quickly into other elements. Their applications are primarily confined to research purposes in nuclear physics and related fields. Understanding these less prevalent isotopes contributes to a more comprehensive picture of silver's nuclear properties.
Applications Leveraging Silver's Properties
The unique properties of silver, including its excellent electrical and thermal conductivity, antimicrobial effects, and aesthetic appeal, have led to numerous applications across diverse fields.
Electrical and Electronic Applications
Silver's high electrical conductivity makes it crucial in various electronic components, including:
- Electrical contacts and connectors: Silver's ability to conduct electricity efficiently with minimal resistance is vital for reliable electrical connections.
- Printed circuit boards: Silver-based inks and pastes are used in the fabrication of printed circuit boards (PCBs), enabling the creation of complex electronic circuitry.
- Solar cells: Silver's high reflectivity and conductivity contribute to the efficiency of certain types of solar cells.
Medical and Antimicrobial Applications
Silver's antimicrobial properties have been known for centuries, and modern research continues to explore its therapeutic potential.
- Wound dressings: Silver-containing dressings are used to prevent bacterial infections in wounds.
- Antimicrobial coatings: Silver-coated surfaces are employed in medical devices and other applications to inhibit bacterial growth.
- Water purification: Silver nanoparticles are used as an antimicrobial agent in water purification systems.
Other Notable Applications
Beyond the applications mentioned above, silver finds utility in various other domains:
- Photography: Silver halide compounds were historically crucial in photographic film and paper.
- Catalysis: Silver acts as a catalyst in various chemical reactions.
- Jewelry and ornaments: Silver's beauty and malleability make it a popular choice in jewelry and decorative items.
- Mirrors and coatings: Silver's high reflectivity makes it ideal for producing mirrors and reflective coatings.
Conclusion: The Neutron Count and Silver's Diverse Roles
The seemingly simple question of "How many neutrons does silver have?" reveals a more complex story of isotopic diversity and the consequential influence on silver's properties and applications. Understanding the existence of both ¹⁰⁷Ag (60 neutrons) and ¹⁰⁹Ag (62 neutrons) with their nearly equal natural abundance is key to appreciating silver's unique characteristics. From its widespread use in electronics and medicine to its aesthetic value in jewelry, silver's multifaceted applications are deeply intertwined with its fundamental nuclear properties. The continuing research into silver's isotopes and their behavior further enhances our understanding of this fascinating and valuable metal. The next time you encounter silver, remember the intricate nuclear processes that shape this remarkable element.
Latest Posts
Latest Posts
-
How Many Inches Is 44 Centimeters
Apr 07, 2025
-
What Is 0 25 As A Percent
Apr 07, 2025
-
1 Meter 92 Cm In Feet
Apr 07, 2025
-
How Many Minutes Is 14 Hours
Apr 07, 2025
-
3 Is What Percent Of 40
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Silver Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.