How Many Orbitals Are In The D Sublevel
Kalali
Mar 17, 2025 · 5 min read
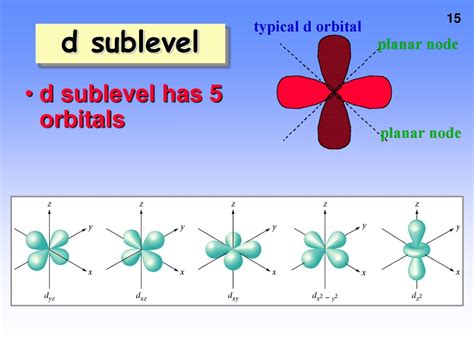
Table of Contents
How Many Orbitals are in the d Sublevel? A Deep Dive into Atomic Structure
Understanding atomic structure is fundamental to grasping the principles of chemistry. One crucial aspect of this understanding involves the arrangement of electrons within an atom, which is dictated by the quantum mechanical model. A key component of this model are sublevels, including the d sublevel, which plays a vital role in the properties of transition metals and many chemical reactions. This article delves deep into the question: how many orbitals are in the d sublevel? We'll explore the underlying quantum numbers, visualize the shapes of d orbitals, and examine their significance in chemistry.
Understanding Quantum Numbers and Electron Configuration
Before we can determine the number of orbitals in the d sublevel, we need to review the fundamental concepts of quantum numbers. These numbers describe the properties of atomic orbitals and the electrons within them. The four main quantum numbers are:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can take on positive integer values (n = 1, 2, 3, ...). A higher n value indicates a higher energy level and a larger orbital.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and the subshells within a principal energy level. It can have integer values ranging from 0 to n - 1. The values of l correspond to different subshells: l = 0 (s subshell), l = 1 (p subshell), l = 2 (d subshell), l = 3 (f subshell), and so on.
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can have integer values ranging from -l to +l, including 0. For example, if l = 1 (p subshell), ml can be -1, 0, or +1, representing three p orbitals oriented along the x, y, and z axes.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can only have two values: +1/2 (spin up) or -1/2 (spin down). This means each orbital can hold a maximum of two electrons with opposite spins (Pauli Exclusion Principle).
Determining the Number of d Orbitals
Now, let's apply this knowledge to the d sublevel. The d sublevel is characterized by an azimuthal quantum number of l = 2. The magnetic quantum number (ml) can therefore take on values from -2 to +2, which are: -2, -1, 0, +1, +2.
This means there are five possible values for ml when l = 2. Each unique value of ml represents a different d orbital. Therefore, there are five d orbitals in the d sublevel.
Visualizing the d Orbitals
It's important to visualize the shapes of these five d orbitals. They are more complex than the s and p orbitals. Four of the d orbitals have a cloverleaf shape with four lobes, while the fifth (d<sub>z²</sub>) has a different shape with two lobes along the z-axis and a ring in the xy-plane. These distinct shapes and orientations are crucial for understanding the directional nature of chemical bonds involving d orbitals.
The five d orbitals are commonly designated as:
- d<sub>xy</sub>: The lobes lie between the x and y axes.
- d<sub>xz</sub>: The lobes lie between the x and z axes.
- d<sub>yz</sub>: The lobes lie between the y and z axes.
- d<sub>x²-y²</sub>: The lobes lie along the x and y axes.
- d<sub>z²</sub>: The lobes lie along the z-axis, with a ring in the xy-plane.
The Significance of d Orbitals in Chemistry
The presence of five d orbitals has profound implications for the properties of elements, particularly transition metals. Transition metals are characterized by partially filled d orbitals. The electrons in these orbitals participate in a wide range of chemical reactions and contribute to the characteristic properties of these elements, including:
-
Variable Oxidation States: The ability of transition metals to exhibit multiple oxidation states is directly related to the presence of partially filled d orbitals. Electrons from these orbitals can be easily lost or gained, leading to different oxidation states.
-
Formation of Colored Compounds: The d orbitals are involved in the absorption and emission of light, which leads to the characteristic colors observed in many transition metal compounds. The energy difference between d orbitals influences the wavelengths of light absorbed or emitted.
-
Catalytic Activity: Transition metals and their compounds are often excellent catalysts because their d orbitals can readily accept or donate electrons, facilitating chemical reactions.
-
Magnetic Properties: The presence of unpaired electrons in d orbitals results in paramagnetism, meaning the compounds are attracted to magnetic fields. If all d electrons are paired, the compound exhibits diamagnetism, meaning it is repelled by magnetic fields.
Beyond the Basics: d-Orbital Hybridization
The concept of orbital hybridization further expands our understanding of the role of d orbitals in chemical bonding. In some molecules, d orbitals can hybridize with s and p orbitals to form hybrid orbitals with different shapes and energies. This is particularly important in explaining the geometry and bonding in transition metal complexes. Examples of d-orbital hybridization include sp³d, sp³d², and d²sp³ hybridization schemes, which lead to different coordination geometries around the central metal ion.
d Orbitals and Spectroscopic Techniques
The energy levels and electronic transitions involving d orbitals can be studied using various spectroscopic techniques, such as UV-Vis spectroscopy and X-ray photoelectron spectroscopy (XPS). These techniques provide valuable information about the electronic structure of transition metal complexes and help researchers understand their chemical behavior.
Conclusion: The Importance of Understanding d Orbitals
In summary, the d sublevel contains five orbitals, each with a unique orientation in space. These orbitals are crucial for understanding the properties and behavior of transition metals and their compounds. The presence of five d orbitals allows for variable oxidation states, the formation of colored compounds, catalytic activity, and diverse magnetic properties. Furthermore, d orbital hybridization plays a vital role in explaining the bonding and geometry of transition metal complexes. A thorough understanding of d orbitals is therefore essential for anyone studying chemistry, especially inorganic and physical chemistry. By exploring the quantum numbers and visualizing the orbital shapes, we gain a deeper appreciation of the complexities and beauty of atomic structure and its impact on the chemical world. The number five, representing the five d orbitals, is a fundamental constant influencing a vast array of chemical phenomena.
Latest Posts
Latest Posts
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
-
What Is Melting Point Of Glass
Mar 17, 2025
-
Cuanto Es El 30 Por Ciento De 500
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Orbitals Are In The D Sublevel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
