How Many Protons Are In An Atom Of Bismuth
Kalali
Mar 12, 2025 · 6 min read
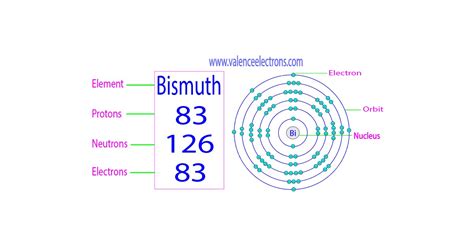
Table of Contents
How Many Protons Are in an Atom of Bismuth? Unveiling the Atomic Heart of a Curious Element
Bismuth. The name itself conjures images of iridescent crystals and unique properties. But beyond its captivating visual appeal lies a fundamental truth about its atomic structure: the number of protons it possesses. This seemingly simple question opens the door to a deeper understanding of atomic structure, periodic trends, and the very nature of matter. So, how many protons are in an atom of bismuth? The answer, simply put, is 83. But let's delve much deeper into why this number is so significant and what it reveals about this fascinating element.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we focus solely on bismuth, let's establish a foundational understanding of atomic structure. Every atom, the fundamental building block of matter, consists of three primary subatomic particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element. Each element has a unique number of protons, and this number is called the atomic number.
-
Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. Neutrons contribute to the atom's mass but not its charge. Isotopes of an element have the same number of protons but differ in their number of neutrons.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. Electrons are much lighter than protons and neutrons and determine the atom's chemical properties and its ability to form bonds with other atoms.
The number of protons, neutrons, and electrons within an atom dictates its properties. The arrangement of electrons dictates how an atom will interact with other atoms. Understanding this fundamental structure is crucial to understanding the behavior of all matter.
Bismuth's Atomic Number: The Defining Characteristic
Bismuth (Bi), a post-transition metal found in the p-block of the periodic table, holds the atomic number 83. This single number, 83, unequivocally defines bismuth. It means that every atom of bismuth, regardless of its isotopic variation, contains 83 protons within its nucleus. This fundamental characteristic dictates bismuth's position on the periodic table, its chemical behavior, and its unique properties.
This number, 83, isn't just an arbitrary value; it reflects its place within the periodic system and provides crucial insights into its reactivity and interactions with other elements. Its atomic number places it precisely where it belongs, distinguishing it from all other elements.
Isotopes of Bismuth: Variations in Neutron Count
While the number of protons remains constant at 83 for all bismuth atoms, the number of neutrons can vary. These variations create different isotopes of bismuth. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutron number alters the atom's mass but does not affect its chemical properties significantly.
Bismuth primarily exists as a single stable isotope, bismuth-209 (²⁰⁹Bi). This isotope contains 83 protons and 126 neutrons (83 + 126 = 209, representing the mass number). While other bismuth isotopes have been synthesized, they are radioactive and decay into other elements over time. This means that most bismuth found in nature is the stable ²⁰⁹Bi isotope.
Bismuth's Properties: A Consequence of Atomic Structure
The unique properties of bismuth are a direct consequence of its atomic structure, particularly its 83 protons and the resulting electron configuration. Some of its notable properties include:
-
Diamagnetism: Bismuth is strongly diamagnetic, meaning it repels magnetic fields. This property stems from the specific arrangement of its electrons.
-
Low Toxicity (relatively): Compared to other heavy metals, bismuth is relatively non-toxic. This makes it suitable for use in various applications, including pharmaceuticals. However, it's important to remember that even relatively non-toxic substances can have negative effects at high levels of exposure.
-
High Density: Bismuth has a high density, exceeding that of many common metals. This property contributes to its use in certain applications where weight is a factor.
-
Semimetallic Character: Bismuth exhibits properties of both metals and nonmetals, displaying a semi-metallic character. This dual nature influences its electrical and thermal conductivity.
-
Brittle Nature: Unlike many metals, bismuth is quite brittle and easily shattered. This characteristic is related to its crystalline structure and bonding behavior.
-
Unique Crystal Structures: Bismuth forms striking iridescent crystals, often displaying a rainbow-like effect due to the interference of light reflecting off its layered crystal structure.
Bismuth's Applications: Utilizing its Unique Properties
The unique properties of bismuth stemming from its atomic structure lead to its diverse applications across various industries:
-
Pharmaceuticals: Bismuth subsalicylate is a common active ingredient in medications used to treat diarrhea and upset stomachs. Its relatively low toxicity makes it suitable for medicinal use.
-
Cosmetics: Bismuth oxychloride is used as a pearlescent pigment in cosmetics, giving products a shimmering effect.
-
Alloys: Bismuth is added to various alloys to lower their melting points, improving their castability.
-
Fire Safety: Bismuth-based compounds are being explored for use in fire suppression systems due to their ability to generate less toxic fumes compared to traditional halon-based extinguishers.
-
Nuclear Research: Bismuth's properties also have applications in nuclear research and technology, specifically related to its interaction with neutrons.
Bismuth in the Periodic Table: Its Position and Trends
Bismuth's position in the periodic table, as element number 83, allows us to understand its properties within the context of periodic trends. It's a member of Group 15 (also known as the pnictogens) which includes nitrogen, phosphorus, arsenic, and antimony. These elements share some similar properties, but bismuth's properties are significantly influenced by its high atomic number and the relativistic effects influencing the behavior of its electrons.
The periodic trends demonstrate a progression of properties as we move down Group 15. The metallic character increases, while the electronegativity decreases. Bismuth shows the most pronounced metallic character among its group members.
Conclusion: The Significance of 83 Protons
The simple answer, 83 protons, represents a crucial piece of information regarding bismuth. This number isn't merely a numerical identifier; it's the fundamental characteristic that defines bismuth as a unique element. It dictates its position on the periodic table, its atomic structure, its chemical behavior, and, ultimately, its remarkable properties. Understanding the number of protons in an atom, especially in the context of bismuth, unveils a deeper appreciation for the structure of matter and the underlying principles of chemistry and physics. The seemingly simple question—how many protons are in an atom of bismuth?—leads to a rich and fascinating exploration of this unique and intriguing element.
Latest Posts
Latest Posts
-
Cuanto Es 5 1 En Metros
May 09, 2025
-
What Do The Inner Planets All Have In Common
May 09, 2025
-
What Is The Least Common Multiple Of 7 And 12
May 09, 2025
-
10 By The Power Of 8
May 09, 2025
-
What Is The Lcm For 7 And 9
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Are In An Atom Of Bismuth . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.