How Many Valence Electrons Are In Calcium
Kalali
Mar 11, 2025 · 6 min read
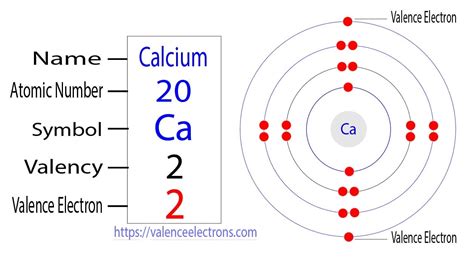
Table of Contents
How Many Valence Electrons are in Calcium? A Deep Dive into Atomic Structure
Understanding the number of valence electrons in an element is fundamental to comprehending its chemical behavior and reactivity. This article will delve into the specifics of calcium (Ca), exploring its electronic configuration, its position in the periodic table, and how this all relates to its valence electron count. We'll also look at the implications of this electron count for calcium's bonding properties and its role in various chemical processes. By the end, you'll have a thorough understanding of calcium's valence electrons and their significance.
Understanding Valence Electrons
Before we dive into calcium specifically, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely bound to the nucleus and, consequently, are the ones most involved in chemical bonding. They determine an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons typically ranges from one to eight.
Calcium's Position in the Periodic Table
The periodic table is a powerful tool for predicting the properties of elements, including the number of valence electrons. Calcium (Ca) is located in Group 2 (also known as Group IIA or the alkaline earth metals) and Period 4 of the periodic table. Its atomic number is 20, indicating it has 20 protons and, in a neutral atom, 20 electrons.
Determining Calcium's Electronic Configuration
To determine the number of valence electrons in calcium, we need to understand its electronic configuration. This describes how the electrons are distributed among the various energy levels and sublevels within the atom. The electronic configuration of calcium is:
1s²2s²2p⁶3s²3p⁶4s²
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), in the s sublevel.
- 2s²: Two electrons in the second energy level (n=2), in the s sublevel.
- 2p⁶: Six electrons in the second energy level (n=2), in the p sublevel.
- 3s²: Two electrons in the third energy level (n=3), in the s sublevel.
- 3p⁶: Six electrons in the third energy level (n=3), in the p sublevel.
- 4s²: Two electrons in the fourth energy level (n=4), in the s sublevel.
Identifying Valence Electrons in Calcium
The outermost energy level for calcium is the fourth energy level (n=4). This level contains two electrons in the 4s sublevel. Therefore, calcium has two valence electrons.
This aligns perfectly with its position in Group 2 of the periodic table. Elements in Group 2 are characterized by having two valence electrons. This common characteristic explains their similar chemical properties.
Implications of Calcium's Two Valence Electrons
The presence of two valence electrons profoundly influences calcium's chemical behavior. Here are some key implications:
1. Ionic Bonding:
Calcium readily loses its two valence electrons to achieve a stable octet (a full outer shell) configuration. This results in the formation of a Ca²⁺ ion, a positively charged ion. This tendency to lose electrons makes calcium highly reactive, especially with electronegative elements like oxygen and chlorine. The ionic bonds formed are strong and lead to the formation of stable ionic compounds. For example, calcium readily reacts with oxygen to form calcium oxide (CaO), a process involving the transfer of electrons.
2. Metallic Bonding:
Calcium, being a metal, exhibits metallic bonding. In metallic bonding, valence electrons are delocalized and shared among a "sea" of electrons within the metal lattice. This allows for good electrical and thermal conductivity, as these delocalized electrons are free to move. The relatively weak attraction between the calcium ions and the delocalized electrons contributes to calcium's malleability and ductility.
3. Reactivity:
The two valence electrons contribute to calcium's relatively high reactivity. It readily reacts with water, acids, and oxygen, producing heat in the process. This reactivity is a defining characteristic of the alkaline earth metals.
4. Biological Importance:
Calcium's chemical properties, largely dictated by its two valence electrons, are crucial for various biological processes. It plays a vital role in bone and tooth formation, muscle contraction, nerve impulse transmission, and blood clotting. The calcium ions (Ca²⁺) interact with various biomolecules, facilitating these essential biological functions.
Calcium's Chemical Reactions: A Closer Look
Let's examine a few examples of calcium's reactions to further illustrate the role of its valence electrons:
1. Reaction with Water:
Calcium reacts vigorously with water to produce calcium hydroxide (Ca(OH)₂) and hydrogen gas (H₂). The two valence electrons of calcium are transferred to two hydrogen atoms, forming hydrogen gas and leaving behind a Ca²⁺ ion which bonds with two hydroxide ions (OH⁻). The reaction is exothermic, releasing heat.
2. Reaction with Oxygen:
Calcium readily reacts with oxygen in the air to form calcium oxide (CaO), also known as quicklime. Again, the two valence electrons are transferred, this time to an oxygen atom, forming a stable ionic compound.
3. Reaction with Acids:
Calcium reacts with acids like hydrochloric acid (HCl) to produce calcium chloride (CaCl₂), hydrogen gas (H₂), and heat. The calcium atoms donate their two valence electrons to the hydrogen ions (H⁺) in the acid, forming hydrogen gas and leaving behind the Ca²⁺ ion, which then combines with chloride ions (Cl⁻).
Beyond Valence Electrons: Other Contributing Factors to Calcium's Properties
While valence electrons are the primary determinant of an element's chemical behavior, other factors also contribute to calcium's overall properties. These include:
- Atomic size: Calcium has a relatively large atomic radius compared to other elements in its period, influencing its reactivity and bonding characteristics.
- Ionization energy: The energy required to remove valence electrons from calcium is relatively low, consistent with its tendency to form positive ions.
- Electronegativity: Calcium has a low electronegativity, meaning it has a weak attraction for electrons in a chemical bond, preferring to lose electrons rather than gain them.
Conclusion: Calcium's Valence Electrons and Their Significance
The presence of two valence electrons is the cornerstone of calcium's chemical behavior and its crucial role in various biological and industrial applications. Understanding its electronic configuration and the implications of these two outermost electrons is essential for grasping its reactivity, bonding patterns, and its unique properties. Its relatively high reactivity, its tendency to form ionic bonds, and its essential role in biological systems all stem from the fundamental characteristic of having two valence electrons. This detailed exploration underscores the importance of valence electrons in understanding the behavior of elements and their interactions within the chemical world. From its vigorous reactions with water and acids to its critical biological roles, calcium's properties are a direct consequence of its two readily available valence electrons. The periodic table serves as a valuable tool for predicting and understanding these properties, cementing the importance of this seemingly simple number in the larger context of chemistry and beyond.
Latest Posts
Latest Posts
-
How Much Is 1 1 4 Oz
Mar 12, 2025
-
1 1 2 Inch In Mm
Mar 12, 2025
-
In A Solution It Is Dissolving Medium
Mar 12, 2025
-
What Tissue Has Lacunae Calcium Salts And Blood Vessels
Mar 12, 2025
-
How Much Is 1 1 2 Cup
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Calcium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
