How Many Valence Electrons Are In Potassium
Kalali
Mar 11, 2025 · 6 min read
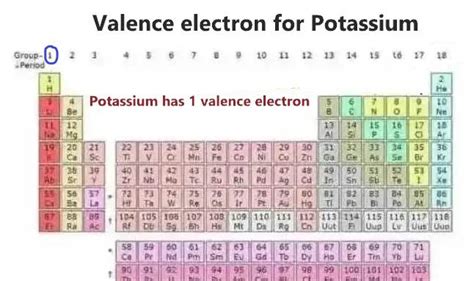
Table of Contents
How Many Valence Electrons Are in Potassium? A Deep Dive into Atomic Structure
Potassium, a crucial element for human health and a key player in various industrial applications, holds a fascinating position within the periodic table. Understanding its atomic structure, particularly the number of valence electrons, is key to comprehending its reactivity and chemical behavior. This comprehensive article will explore this topic in detail, delving into the underlying principles of electron configuration and atomic orbitals to definitively answer the question: how many valence electrons are in potassium?
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we pinpoint the number of valence electrons in potassium, let's establish a clear understanding of what valence electrons are and why they are so important. Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are the furthest from the atom's nucleus and, consequently, are the most loosely bound. This loose binding makes them highly susceptible to interaction with other atoms, ultimately dictating an element's chemical properties and reactivity.
It's the valence electrons that participate in chemical bonding – the forces that hold atoms together to form molecules and compounds. Atoms strive to achieve a stable electron configuration, often resembling the noble gases (Group 18 elements) which have full outer electron shells. This tendency to gain, lose, or share electrons to achieve stability is the driving force behind chemical reactions.
Potassium's Place in the Periodic Table: A Clue to its Electron Configuration
Potassium (K), with atomic number 19, resides in Group 1 (also known as the alkali metals) of the periodic table. The periodic table's organization is no accident; it reflects the underlying patterns in electron configurations. Group 1 elements are characterized by having one valence electron in their outermost shell. This single valence electron is easily lost, leading to the formation of a +1 ion (K⁺). This characteristic explains potassium's high reactivity and its tendency to form ionic compounds.
Determining Potassium's Electron Configuration: A Step-by-Step Approach
To understand why potassium has only one valence electron, we need to delve into its electron configuration. This describes how electrons are distributed among different energy levels and sublevels within the atom. The electron configuration is determined by filling orbitals according to the Aufbau principle (filling lower energy levels first), Hund's rule (maximizing unpaired electrons), and the Pauli exclusion principle (each orbital holding a maximum of two electrons with opposite spins).
The electron configuration of potassium (K, atomic number 19) is: 1s²2s²2p⁶3s²3p⁶4s¹.
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), in the s orbital (l=0).
- 2s²: Two electrons in the second energy level (n=2), in the s orbital.
- 2p⁶: Six electrons in the second energy level, in the p orbitals (l=1). There are three p orbitals, each holding a maximum of two electrons.
- 3s²: Two electrons in the third energy level (n=3), in the s orbital.
- 3p⁶: Six electrons in the third energy level, in the p orbitals.
- 4s¹: One electron in the fourth energy level (n=4), in the s orbital. This is the outermost shell.
The Significance of the 4s Orbital: Why Only One Valence Electron?
Notice the final part of potassium's electron configuration: 4s¹. This indicates that the outermost energy level (n=4) contains only one electron. This lone electron resides in the 4s orbital and, therefore, constitutes potassium's single valence electron. The filled inner shells (1s², 2s², 2p⁶, 3s², 3p⁶) are energetically stable and do not participate in chemical bonding.
The fact that potassium readily loses this single valence electron to achieve a stable, noble gas configuration (like Argon) is fundamental to its chemical behavior. The resulting K⁺ ion is highly stable and readily participates in ionic bonding with anions (negatively charged ions) to form ionic compounds.
Potassium's Reactivity: A Consequence of its Single Valence Electron
The presence of only one valence electron directly contributes to potassium's high reactivity. Potassium is a highly reactive alkali metal, readily reacting with water, oxygen, and halogens. The ease with which potassium loses its valence electron is a major factor contributing to this reactivity. This single valence electron is easily ionized, meaning it’s easily removed, leaving behind a positively charged ion (K⁺). This makes potassium a powerful reducing agent. It readily donates its electron to other atoms, causing them to be reduced.
Applications of Potassium: Harnessing its Reactivity
Potassium's unique properties, stemming from its single valence electron, make it essential in various applications:
-
Fertilizers: Potassium is a crucial macronutrient for plant growth, playing a vital role in enzyme activation, protein synthesis, and carbohydrate metabolism. Potassium-containing fertilizers are extensively used in agriculture to enhance crop yields.
-
Human health: Potassium is an essential electrolyte in the human body. It helps regulate fluid balance, muscle contractions, nerve impulses, and blood pressure. Potassium deficiency can lead to various health problems.
-
Industrial uses: Potassium compounds find applications in various industrial processes, including the production of soap, glass, and certain types of cement. Potassium hydroxide (KOH) is a strong base used in various chemical reactions.
-
Photography: Potassium salts, notably potassium bromide (KBr), have historical significance in photography as part of photographic emulsions.
Beyond the Basics: A Deeper Look at Atomic Orbitals
To further enhance our understanding, let's briefly explore the concept of atomic orbitals. Atomic orbitals are regions of space around the nucleus where there's a high probability of finding an electron. These orbitals have specific shapes and energy levels. The s orbitals are spherical, while the p orbitals are dumbbell-shaped. The 4s orbital, where potassium's valence electron resides, is larger and has a higher energy level than the inner orbitals. This higher energy level contributes to the ease with which the valence electron is lost in chemical reactions.
Conclusion: One Valence Electron, Countless Applications
In conclusion, potassium possesses one valence electron, located in the 4s orbital of its outermost electron shell. This single valence electron is the key to understanding potassium's chemical properties, reactivity, and widespread applications. Its tendency to lose this electron to achieve a stable noble gas configuration drives its participation in ionic bonding and numerous chemical reactions. This understanding of atomic structure and electron configuration is not only fundamental to chemistry but also crucial for appreciating the role of potassium in various scientific, industrial, and biological contexts. From fertilizers to human health to industrial processes, the influence of this single valence electron is vast and significant.
Latest Posts
Latest Posts
-
How Far Is 0 4 Miles To Walk
Jul 12, 2025
-
What Is 20 Percent Of 800 000
Jul 12, 2025
-
Words That Start With Y In Science
Jul 12, 2025
-
Prevent An Expressway Emergency By Merging Without
Jul 12, 2025
-
How Many Grams Of Sugar In A Pound
Jul 12, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.