How Many Valence Electrons Do The Halogens Possess
Kalali
Mar 17, 2025 · 5 min read
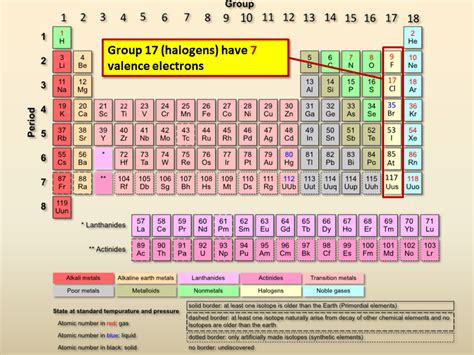
Table of Contents
How Many Valence Electrons Do the Halogens Possess? A Deep Dive into Group 17
The halogens, a vibrant and reactive group of nonmetals, hold a special place in the periodic table. Their unique chemical properties are directly tied to their electronic configuration, specifically the number of valence electrons they possess. Understanding this fundamental aspect is key to comprehending their behavior and the diverse applications they find in various fields. This article delves deep into the fascinating world of halogens, explaining not only how many valence electrons they have but also the implications of this characteristic on their reactivity and bonding.
Understanding Valence Electrons
Before we dive into the specifics of halogens, let's clarify the concept of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely held and therefore are primarily involved in chemical bonding. The number of valence electrons determines an element's chemical reactivity and the types of bonds it can form. Atoms tend to react in ways that achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to attain a full outermost shell (typically eight electrons, following the octet rule).
The Halogens: A Family of Reactive Nonmetals
The halogens (Group 17, or VIIA) are a family of nonmetals that exhibit remarkably similar chemical properties. This family includes:
- Fluorine (F): The most reactive and electronegative element.
- Chlorine (Cl): A crucial element in many industrial processes and disinfectants.
- Bromine (Br): The only non-metal liquid at room temperature.
- Iodine (I): Used in antiseptic solutions and in the production of certain pharmaceuticals.
- Astatine (At): A highly radioactive and unstable element, making its study challenging.
All these elements share a common characteristic: they possess seven valence electrons. This is a crucial detail that governs their chemistry and reactivity.
Why Seven Valence Electrons Matter: The Drive for Stability
With seven valence electrons, halogens are only one electron short of achieving a stable, noble gas configuration. This inherent instability drives their strong tendency to gain an electron, forming a negatively charged ion (anion) with a -1 charge. This process is known as reduction, where the halogen atom gains an electron, decreasing its oxidation state.
This drive for stability explains their high electronegativity. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Halogens have high electronegativity because of their strong pull on electrons due to their almost-complete outermost shell. This strong pull often results in the formation of ionic bonds with metals.
Ionic Bonding: A Consequence of Seven Valence Electrons
When a halogen interacts with a metal (like sodium or potassium, which readily lose electrons), the metal atom donates an electron to the halogen atom. This electron transfer creates an ionic bond, forming a stable ionic compound. For example, sodium chloride (NaCl), common table salt, is formed through this process: Sodium (Na) loses one electron to become Na⁺, and chlorine (Cl) gains that electron to become Cl⁻, forming a stable ionic lattice structure.
Covalent Bonding: Sharing Electrons to Achieve Stability
Halogens can also form covalent bonds, sharing electrons with other nonmetals, particularly other halogens or carbon. This occurs when the electronegativity difference between the atoms is relatively small. For instance, chlorine can form diatomic molecules (Cl₂), where two chlorine atoms share one electron pair, completing their octets. This sharing allows both chlorine atoms to effectively have eight valence electrons. Similarly, halogenated organic compounds, widely used in various industrial applications and some refrigerants, are formed through covalent bonds between carbon and halogen atoms.
The Implications of Seven Valence Electrons: Reactivity and Applications
The seven valence electrons of halogens have profound implications for their reactivity and the wide range of applications they find:
Reactivity: A Dominant Feature
The high reactivity of halogens is a direct result of their eagerness to gain that one missing electron. Fluorine, being the most electronegative, is the most reactive halogen. Reactivity generally decreases as you move down the group (F > Cl > Br > I > At). This trend is linked to the increasing atomic size and decreasing electronegativity down the group. Larger atoms have their valence electrons further from the nucleus, making them less tightly held and thus less readily accepted.
Applications: Diverse and Extensive
The unique properties of halogens, dictated by their seven valence electrons, lead to a broad spectrum of applications across various industries:
- Fluorine: Used in refrigerants (although some are now phased out due to environmental concerns), Teflon coatings, and in the production of uranium hexafluoride (UF₆) for nuclear fuel enrichment.
- Chlorine: Widely used as a disinfectant in water treatment and swimming pools, in the production of PVC plastics, and as a bleaching agent.
- Bromine: Used in flame retardants, pesticides, and in the production of certain dyes.
- Iodine: A crucial component in thyroid hormones, and used as an antiseptic and in photography.
- Astatine: Its extremely short half-life limits its practical applications, primarily restricted to research in nuclear medicine.
Beyond the Octet Rule: Exceptions and Nuances
While the octet rule serves as a useful guideline, there are exceptions, particularly with halogens. Some halogen compounds may exist where the central halogen atom has more or less than eight electrons in its valence shell. This can happen in situations involving hypervalency or electron deficiency. These exceptions highlight the complex interplay of factors influencing chemical bonding.
Conclusion: Seven Valence Electrons – A Defining Characteristic
The seven valence electrons possessed by halogens are fundamental to their chemical behavior. This characteristic drives their high reactivity, electronegativity, and the formation of various types of bonds, leading to a vast array of applications in numerous fields. Understanding this fundamental aspect is crucial for appreciating the importance of halogens in our daily lives, from the water we drink to the materials used in countless products. Furthermore, continued research into the chemistry of halogens reveals ever-more nuanced insights into the complexities of chemical bonding and reactivity. The simplicity of stating that halogens have seven valence electrons belies the richness and complexity of the chemical world they inhabit.
Latest Posts
Latest Posts
-
What Fish Has Fins And Scales
Mar 17, 2025
-
What Is The Melting Point For Glass
Mar 17, 2025
-
What Is The Decimal Of 6 8
Mar 17, 2025
-
Cuanto Es El 30 Por Ciento De 1000
Mar 17, 2025
-
Which Of The Following Substances Is An Arrhenius Acid
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Do The Halogens Possess . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
