How Many Valence Electrons Does Calcium Ca Have
Kalali
Mar 17, 2025 · 6 min read
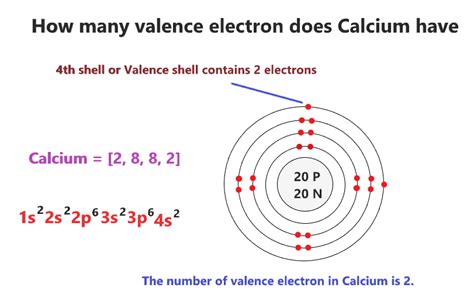
Table of Contents
How Many Valence Electrons Does Calcium (Ca) Have? A Deep Dive into Calcium's Electronic Structure
Calcium, a vital element for human health and a cornerstone of numerous industrial applications, holds a fascinating position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is key to grasping its chemical behavior and reactivity. This comprehensive guide will not only answer the central question – how many valence electrons does calcium have? – but also delve into the underlying principles of electron configuration, explaining why calcium behaves the way it does.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we pinpoint the number of valence electrons in calcium, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and, therefore, are the primary participants in chemical bonding. They determine an element's reactivity and the types of chemical bonds it can form – ionic, covalent, or metallic. The number of valence electrons directly influences an atom's propensity to gain, lose, or share electrons to achieve a stable electron configuration, usually a full outer shell (often following the octet rule, aiming for eight electrons).
Calcium's Position in the Periodic Table: A Clue to its Valence Electrons
Calcium (Ca) resides in Group 2 (also known as Group IIA or the alkaline earth metals) of the periodic table. The group number itself offers a significant clue to the number of valence electrons. Elements within the same group share similar chemical properties largely due to having the same number of valence electrons. Group 2 elements are characterized by having two valence electrons.
Calcium's Electron Configuration: Unraveling the Mystery
To definitively determine the number of valence electrons in calcium, we must examine its electron configuration. The electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. Calcium's atomic number is 20, indicating it has 20 protons and, in a neutral atom, 20 electrons. Its electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), in the s sublevel.
- 2s² 2p⁶: Eight electrons in the second energy level (n=2), with two in the s sublevel and six in the p sublevel.
- 3s² 3p⁶: Eight electrons in the third energy level (n=3), with two in the s sublevel and six in the p sublevel.
- 4s²: Two electrons in the fourth energy level (n=4), in the s sublevel.
The outermost energy level for calcium is the fourth energy level (n=4), which contains two electrons in the 4s sublevel. Therefore, calcium possesses two valence electrons.
Why Two Valence Electrons? The Significance of the Octet Rule (and its exceptions)
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons, resembling the stable electron configuration of noble gases. While the octet rule serves as a useful guideline, it's not universally applicable. For elements in the first three periods (rows) of the periodic table, achieving a full outer shell usually means eight electrons. However, for higher periods, this isn't always the case.
Calcium, despite not achieving a full octet by losing its two valence electrons, achieves a stable configuration. By losing these two electrons, it attains the electron configuration of Argon (1s² 2s² 2p⁶ 3s² 3p⁶), a noble gas with a stable, filled outer electron shell. This is a more energetically favorable state, driving calcium's chemical behavior.
Exceptions to the Octet Rule: Why it doesn't always apply
It's crucial to acknowledge that the octet rule has exceptions. Some elements can exist with fewer than eight valence electrons, and some can even exceed eight. Transition metals, including those with d orbitals, and some post-transition metals with accessible d orbitals, often deviate from the octet rule due to the availability of more electron shells and more complex orbital interactions. However, calcium's behavior is a straightforward example of fulfilling the octet principle by achieving a noble gas configuration.
Calcium's Reactivity: A Consequence of its Two Valence Electrons
Calcium's two valence electrons directly contribute to its reactivity. Calcium readily loses these two electrons to form a Ca²⁺ ion, achieving the stable electron configuration of argon. This tendency to lose electrons characterizes its metallic nature and its reactivity with other elements, especially non-metals.
For instance, calcium reacts vigorously with water, producing calcium hydroxide and hydrogen gas. This reaction is driven by calcium's strong desire to lose its valence electrons to form a stable ion. Similarly, calcium reacts readily with oxygen to form calcium oxide, again illustrating its propensity to lose electrons and attain a stable electronic configuration.
Applications of Calcium: A Testament to its Chemical Properties
The properties stemming from calcium's two valence electrons lead to a wide range of applications:
- Biological Roles: Calcium plays a critical role in various biological processes, including muscle contraction, nerve impulse transmission, and bone formation. The ionic form (Ca²⁺) is vital for these processes.
- Metallurgy: Calcium is used as an alloying agent in metallurgy, enhancing the properties of other metals.
- Construction: Calcium compounds, particularly calcium carbonate (limestone and marble), are widely used in construction materials such as cement and plaster.
- Reducing Agent: Its tendency to lose electrons makes it a useful reducing agent in certain chemical processes.
Beyond Valence Electrons: Other Factors Influencing Calcium's Chemistry
While the number of valence electrons is paramount in determining an element's chemical behavior, other factors also play a role. These include:
- Atomic Radius: The size of the atom influences the ease with which it can lose or gain electrons.
- Ionization Energy: The energy required to remove an electron from an atom impacts reactivity.
- Electronegativity: The tendency of an atom to attract electrons in a chemical bond affects the type of bond formed.
Conclusion: The Significance of Calcium's Two Valence Electrons
To reiterate, calcium (Ca) has two valence electrons. This fundamental characteristic shapes its chemical properties, making it a highly reactive alkaline earth metal that readily loses these electrons to form stable ionic compounds. Its reactivity, in turn, underlies its diverse applications in biological systems, industrial processes, and various technological fields. Understanding valence electrons provides a powerful framework for predicting and explaining the chemical behavior of elements, and calcium serves as an excellent example of this principle in action. By understanding its electron configuration and the implications of its two valence electrons, we gain a deeper appreciation for calcium's crucial role in the natural world and human technology.
Latest Posts
Latest Posts
-
How Many Ounces Is 2 3 Cup Of Milk
Mar 17, 2025
-
What Is The Hottest Hour Of The Day
Mar 17, 2025
-
Which Natural Phenomenon Is The Best Example Of Periodic Behavior
Mar 17, 2025
-
What Is 0 08 As A Percentage
Mar 17, 2025
-
How Many Cups In 3 1 2 Quarts
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Calcium Ca Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
