How Many Valence Electrons Does Ne Have
Kalali
Mar 10, 2025 · 5 min read
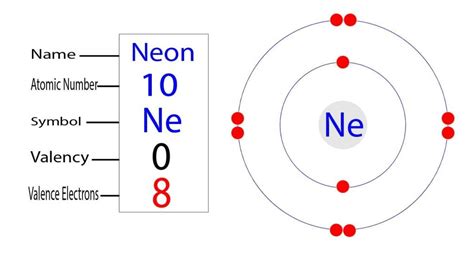
Table of Contents
How Many Valence Electrons Does Neon (Ne) Have? Understanding Noble Gas Electron Configuration
Neon (Ne), a vibrant and inert noble gas, holds a unique position in the periodic table. Its chemical stability and distinctive properties stem directly from its electron configuration, specifically the number of valence electrons it possesses. This article delves deep into the intricacies of neon's electron structure, explaining not only how many valence electrons it has but also why this number is crucial to its chemical behavior and its role in various applications.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we pinpoint neon's valence electron count, let's clarify what valence electrons are and why they're so important. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. Atoms strive for stability, typically achieving it by having a full valence shell, mimicking the electron configuration of noble gases. This drive for stability is the foundation of chemical reactions.
The Significance of a Full Valence Shell
A full valence shell, generally containing eight electrons (the octet rule, with some exceptions for lighter elements), signifies exceptional stability. Atoms with incomplete valence shells readily interact with other atoms to gain, lose, or share electrons to achieve this stable configuration. This electron exchange or sharing forms the basis of chemical bonds—ionic bonds (transfer of electrons) and covalent bonds (sharing of electrons).
Neon's Electron Configuration: Unveiling the Stable Octet
Neon's atomic number is 10, meaning it has 10 protons and 10 electrons in a neutral atom. To understand its electron configuration, we need to follow the Aufbau principle, filling electron shells according to increasing energy levels.
- First Shell (n=1): This shell can hold a maximum of two electrons. Neon's two lowest-energy electrons fill this shell completely.
- Second Shell (n=2): This shell can hold up to eight electrons. Neon's remaining eight electrons fill this shell entirely.
Therefore, neon's complete electron configuration is 1s²2s²2p⁶. This configuration visually represents the distribution of electrons across the different energy levels and subshells.
Identifying Valence Electrons in Neon's Configuration
Now, let's focus on identifying neon's valence electrons. Remember, valence electrons are those in the outermost shell. In neon's case, the outermost shell is the second shell (n=2). This shell contains a total of eight electrons (2s²2p⁶).
Therefore, neon (Ne) has 8 valence electrons.
Why Neon is Inert: The Role of a Full Valence Shell
Neon's eight valence electrons are the key to understanding its inertness (lack of reactivity). With its outermost shell completely filled, neon has achieved maximum stability. It has no tendency to gain, lose, or share electrons to form chemical bonds with other atoms. This complete octet makes neon exceptionally unreactive, a characteristic shared by all noble gases.
Contrasting Neon's Inertness with Other Elements
Compare neon's behavior to that of an element like sodium (Na), which has only one valence electron. Sodium readily loses this electron to achieve a stable octet, forming a positively charged ion (Na⁺). This willingness to lose an electron makes sodium highly reactive, readily participating in chemical reactions. Conversely, neon's stable configuration prevents such interactions.
Applications of Neon: Leveraging its Unique Properties
Despite its inertness, neon finds various applications that capitalize on its unique properties:
1. Neon Lighting: A Vibrant Display of Inertness
Neon's most well-known application is in neon lighting. When an electric current is passed through a neon-filled tube, the neon atoms become excited, causing them to emit a characteristic reddish-orange glow. This light emission arises from the electrons transitioning between energy levels within the neon atoms. The inertness of neon ensures it doesn't react with the electrodes or the surrounding environment, making it safe and reliable for lighting purposes.
2. Cryogenics: Utilizing Neon's Low Boiling Point
Neon possesses an extremely low boiling point (-246.1°C), making it a valuable refrigerant in cryogenic applications. In cryogenic systems, maintaining extremely low temperatures is critical for many processes, including scientific research involving superconductivity or the liquefaction of other gases. Neon's low boiling point allows it to effectively cool these systems.
3. High-Voltage Indicators: Detecting Electrical Discharge
Neon's inertness and electrical conductivity make it suitable for high-voltage indicators. Neon lamps are employed in various electrical equipment to signify the presence of high voltage, serving as a safety measure and a visual indicator of functionality.
4. Laser Technology: Harnessing Neon's Energy Levels
Neon's energy levels are exploited in various laser applications, specifically helium-neon (He-Ne) lasers. These lasers produce a stable and coherent beam of red light used in barcode scanners, laser pointers, and scientific instruments requiring precise and stable light sources.
Neon's Role in Scientific Research
Beyond its practical applications, neon plays a crucial role in scientific research. Its inert nature and well-defined properties make it an ideal component in various studies, including:
- Spectroscopy: Neon's characteristic spectral lines are used as reference points in spectroscopic analyses, allowing researchers to identify other elements or molecules based on their spectral signatures.
- Atmospheric Studies: Neon's presence and isotopic ratios in the atmosphere provide insights into atmospheric processes and the Earth's history.
Conclusion: The Importance of Valence Electrons in Defining Neon's Properties
Neon's eight valence electrons are central to understanding its chemical behavior and its diverse applications. This full valence shell results in its exceptional stability and inertness, characteristics that distinguish it from other elements. This inherent stability is the cornerstone of neon's utilization in lighting, cryogenics, high-voltage indicators, laser technology, and scientific research. By understanding the significance of valence electrons, we can appreciate the unique properties that make neon a versatile element with important roles in both technology and scientific understanding. The simple answer, eight valence electrons, leads to a profound understanding of a noble gas and its impact on our world.
Latest Posts
Latest Posts
-
How Much Is Three Quarts Of Water
Jul 03, 2025
-
How Tall Is A 3 Story Building
Jul 03, 2025
-
Is Miranda Cosgrove Related To Jimmy Fallon
Jul 03, 2025
-
What Is 1 4 Of A 1 4 Cup
Jul 03, 2025
-
What Is 20 Percent Off Of 39 99
Jul 03, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Ne Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.