How Many Valence Electrons In Calcium
Kalali
Mar 13, 2025 · 5 min read
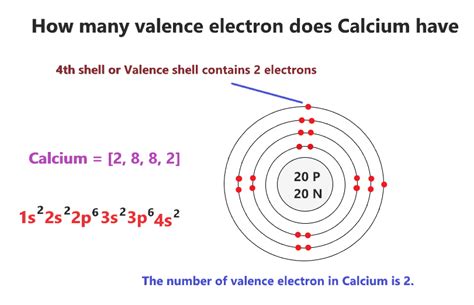
Table of Contents
How Many Valence Electrons Does Calcium Have? A Deep Dive into Atomic Structure
Understanding the number of valence electrons in an element is crucial for predicting its chemical behavior and reactivity. This article delves into the specifics of calcium (Ca), exploring its atomic structure, electron configuration, and ultimately, definitively answering the question: how many valence electrons does calcium have? We'll go beyond a simple answer, examining the concepts that underpin this fundamental aspect of chemistry.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in calcium, let's establish a firm grasp on the concept itself. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the primary players in chemical bonding, dictating how an atom will interact with other atoms to form molecules and compounds. They are responsible for an element's chemical properties and reactivity. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons.
Calcium's Atomic Structure: A Foundation for Understanding Valence Electrons
Calcium, a silvery-white alkaline earth metal, occupies the 20th position on the periodic table. Its atomic number is 20, indicating that a neutral calcium atom possesses 20 protons and 20 electrons. To understand its valence electrons, we must delve into its electron configuration.
Electron Configuration: The Arrangement of Electrons
The electron configuration describes how electrons are distributed among the different energy levels and sublevels within an atom. Calcium's electron configuration is 1s²2s²2p⁶3s²3p⁶4s². This notation tells us:
- 1s²: Two electrons occupy the first energy level (n=1) in the 's' subshell.
- 2s²: Two electrons are in the second energy level (n=2) in the 's' subshell.
- 2p⁶: Six electrons are in the second energy level (n=2) in the 'p' subshell.
- 3s²: Two electrons are in the third energy level (n=3) in the 's' subshell.
- 3p⁶: Six electrons are in the third energy level (n=3) in the 'p' subshell.
- 4s²: Two electrons are in the fourth energy level (n=4) in the 's' subshell.
Identifying the Valence Shell and Valence Electrons
The valence shell is the outermost occupied electron shell. In calcium's electron configuration, the outermost shell is the fourth energy level (n=4). This shell contains two electrons in the 4s subshell. Therefore, calcium has 2 valence electrons.
The Significance of Calcium's Two Valence Electrons
The presence of two valence electrons profoundly influences calcium's chemical properties and behavior. Atoms tend towards stability, often achieved by having a full outermost electron shell (usually 8 electrons, following the octet rule). Calcium readily loses its two valence electrons to achieve a stable electron configuration similar to argon (1s²2s²2p⁶3s²3p⁶), which has a completely filled third shell. This process results in the formation of a Ca²⁺ ion, a positively charged calcium ion.
Chemical Reactions and Bonding
This tendency to lose two electrons explains calcium's reactivity. It readily participates in ionic bonding, where it donates its two valence electrons to a more electronegative atom, such as oxygen or chlorine. The electrostatic attraction between the resulting positively charged calcium ion (Ca²⁺) and the negatively charged ions (e.g., O²⁻, Cl⁻) forms ionic compounds. Examples include calcium oxide (CaO) and calcium chloride (CaCl₂).
Oxidation State and Reactivity
The fact that calcium loses two electrons dictates its oxidation state. Its oxidation state is typically +2, reflecting the loss of two electrons. This positive oxidation state contributes to calcium's reactivity with various substances, making it a relatively reactive metal.
Comparing Calcium to Other Elements
Understanding calcium's valence electrons allows us to compare it with other elements. For instance:
- Magnesium (Mg): Like calcium, magnesium is an alkaline earth metal with two valence electrons. This similarity explains their similar chemical behaviors and reactivity.
- Sodium (Na): Sodium is an alkali metal with only one valence electron. It readily loses this single electron to form a +1 ion. Compared to calcium, sodium is more reactive due to its lower ionization energy.
- Argon (Ar): Argon is a noble gas with a full valence shell (eight electrons). Its stable electron configuration makes it extremely unreactive.
Applications and Significance of Calcium
Calcium's chemical properties, directly linked to its two valence electrons, make it an essential element with numerous applications:
- Biological Roles: Calcium is crucial for biological processes in living organisms. It plays a vital role in bone formation, muscle contraction, nerve impulse transmission, and blood clotting.
- Industrial Uses: Calcium compounds are used extensively in various industries. Calcium carbonate (CaCO₃), for example, is used in cement production, construction materials, and as a dietary supplement. Calcium oxide (CaO), also known as quicklime, is used in steelmaking and wastewater treatment.
- Metallurgy: Calcium is used as an alloying agent in metallurgy to improve the properties of certain metals.
Advanced Concepts Related to Valence Electrons and Calcium
While we've focused on the fundamental aspects, understanding calcium's valence electrons also touches upon more advanced chemical concepts:
- Ionization Energy: The energy required to remove an electron from an atom. Calcium has relatively low ionization energies for its first two electrons, explaining its tendency to lose them.
- Electronegativity: A measure of an atom's ability to attract electrons in a chemical bond. Calcium has low electronegativity, readily losing electrons rather than gaining them.
- Quantum Mechanics: The electron configuration and the very concept of valence electrons are fundamentally based on quantum mechanics, which describes the behavior of electrons in atoms.
Conclusion: Calcium's Two Valence Electrons - A Cornerstone of its Chemistry
In conclusion, calcium has two valence electrons. This seemingly simple fact underpins the entirety of its chemical behavior, reactivity, and crucial biological and industrial applications. Understanding valence electrons is essential for comprehending the fundamental principles of chemistry and predicting the interactions of elements. This deep dive into calcium's atomic structure provides a solid foundation for appreciating the significance of valence electrons and their role in shaping the world around us. Further exploration into these concepts will continue to unveil the intricacies of chemical bonding and reactivity.
Latest Posts
Latest Posts
-
40 Oz Of Water Is How Many Cups
Jul 18, 2025
-
How Many Eighths In A Quarter Pound
Jul 18, 2025
-
Can The Sine Of An Angle Ever Equal 2
Jul 18, 2025
-
How Many Months Is A Hundred Days
Jul 18, 2025
-
Mother And I Or Mother And Me
Jul 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Calcium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.