How Many Valence Electrons In Potassium
Kalali
Mar 22, 2025 · 5 min read
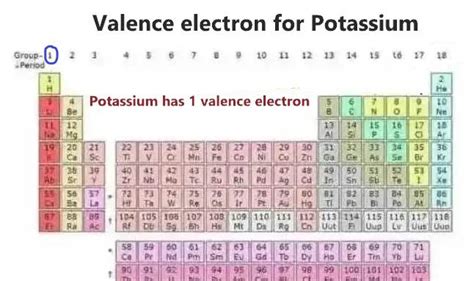
Table of Contents
How Many Valence Electrons Does Potassium Have? A Deep Dive into Atomic Structure
Potassium, a crucial element for human health and various industrial applications, holds a fascinating place in the periodic table. Understanding its electronic configuration, specifically the number of valence electrons, is key to comprehending its chemical reactivity and bonding behavior. This comprehensive guide delves deep into the atomic structure of potassium, explaining not just how many valence electrons it possesses but why it has that specific number, and how this impacts its properties and interactions.
Understanding Valence Electrons: The Key to Chemical Behavior
Before we focus specifically on potassium, let's establish a firm understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the electrons most involved in chemical bonding, determining an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons dictates an atom's tendency to gain, lose, or share electrons to achieve a stable electron configuration, usually a full outermost shell. This stability is often described as following the "octet rule," aiming for eight electrons in the valence shell (except for hydrogen and helium, which strive for two).
Potassium's Position in the Periodic Table: A Clue to its Valence Electrons
Potassium (K), with atomic number 19, resides in Group 1 (also known as Alkali Metals) of the periodic table. The group number itself provides a valuable clue to the number of valence electrons. Group 1 elements are characterized by having one valence electron. This consistent pattern across Group 1 elements is a direct consequence of their electron configurations.
Potassium's Electronic Configuration: Unveiling the Valence Shell
To definitively determine the number of valence electrons in potassium, we need to examine its electronic configuration. This configuration describes how electrons are distributed among the various energy levels (shells) and sublevels (orbitals) within the atom. For potassium, the electronic configuration is: 1s²2s²2p⁶3s²3p⁶4s¹.
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), in the s orbital.
- 2s²: Two electrons in the second energy level (n=2), in the s orbital.
- 2p⁶: Six electrons in the second energy level (n=2), in the three p orbitals.
- 3s²: Two electrons in the third energy level (n=3), in the s orbital.
- 3p⁶: Six electrons in the third energy level (n=3), in the three p orbitals.
- 4s¹: One electron in the fourth energy level (n=4), in the s orbital.
The outermost shell in potassium's configuration is the fourth energy level (n=4). This shell contains only one electron in the 4s orbital. Therefore, potassium has one valence electron.
Why only one valence electron?
The structure of the atom dictates this. Electrons fill energy levels sequentially, with lower energy levels filling before higher ones. The 4s sublevel is filled before the 3d sublevel, leading to the 4s¹ configuration as the outermost shell.
The Chemical Implications of Potassium's Single Valence Electron
Having only one valence electron profoundly impacts potassium's chemical behavior:
-
High Reactivity: Potassium readily loses its single valence electron to achieve a stable octet configuration (like Argon). This makes it highly reactive, especially with electronegative elements such as halogens (e.g., chlorine, bromine). The loss of an electron forms a positively charged ion, K⁺.
-
Ionic Bonding: The tendency to lose its valence electron leads to the formation of ionic bonds. Potassium readily forms ionic compounds with nonmetals, where it transfers its electron to the nonmetal, resulting in electrostatic attraction between the oppositely charged ions. For example, potassium chloride (KCl) is formed through this ionic bonding.
-
Metallic Bonding: Potassium also exhibits metallic bonding in its elemental form. The valence electrons are delocalized, forming a "sea" of electrons that are shared among many potassium atoms. This contributes to potassium's characteristic metallic properties such as conductivity (both electrical and thermal) and malleability.
-
Oxidation State: Because potassium readily loses one electron, its common oxidation state is +1. This is a key characteristic used in predicting its behavior in chemical reactions and compound formation.
Potassium's Role in Biology and Industry
Potassium's unique properties, stemming from its single valence electron, make it crucial in various biological and industrial contexts:
Biological Significance:
-
Electrolyte Balance: Potassium is a crucial electrolyte in living organisms, playing a critical role in maintaining fluid balance, nerve impulse transmission, and muscle contraction. Its ionic form, K⁺, is essential for the proper functioning of cells and overall health.
-
Enzyme Activation: Potassium ions are involved in activating various enzymes, which are crucial biological catalysts.
Industrial Applications:
-
Fertilizers: Potassium compounds are significant components of fertilizers, providing essential nutrients for plant growth. Potassium's role in promoting healthy plant development is well-established.
-
Glass Manufacturing: Potassium compounds are used in the production of specialized glasses, influencing their properties and characteristics.
-
Soap Production: Potassium hydroxide (KOH) is a crucial component in soap manufacturing.
-
Chemical Industry: Potassium compounds find use in various chemical processes and reactions as reactants or catalysts.
Conclusion: The Significance of Understanding Valence Electrons
Understanding the number of valence electrons in an element, particularly potassium in this instance, is fundamental to comprehending its chemical behavior, reactivity, and the types of bonds it forms. Potassium's single valence electron dictates its high reactivity, its tendency to form ionic compounds, its participation in metallic bonding, and its critical role in biological systems and various industrial processes. This knowledge is essential not only for chemists but also for biologists, engineers, and anyone interested in the fascinating world of atoms and their interactions. By grasping the concept of valence electrons and applying it to specific elements like potassium, we can unlock a deeper understanding of the macroscopic properties and behaviors of matter from the microscopic perspective of atomic structure.
Latest Posts
Latest Posts
-
How Long Does It Take To Count To One Billion
Jul 06, 2025
-
Why Did The Kangaroo See A Psychiatrist
Jul 06, 2025
-
How Long Would It Take To Walk To China
Jul 06, 2025
-
Step Up To The Streets Final Dance
Jul 06, 2025
-
How Many Grams Is Half An Oz
Jul 06, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.