How Much Valence Electrons Does Sodium Have
Kalali
Mar 26, 2025 · 5 min read
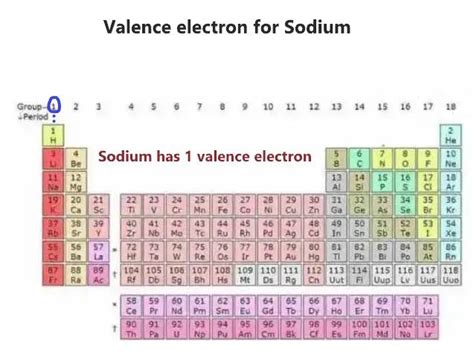
Table of Contents
How Many Valence Electrons Does Sodium Have? A Deep Dive into Atomic Structure
Sodium, a ubiquitous element vital for life and numerous industrial applications, holds a fascinating position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its reactivity and chemical behavior. This article delves deep into the atomic structure of sodium, explaining precisely how many valence electrons it possesses and why this number is so crucial. We'll explore related concepts like electron shells, electron configuration, and the implications of sodium's valence electron count in various chemical contexts.
Understanding Valence Electrons: The Key to Reactivity
Before focusing on sodium specifically, let's establish a solid understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound and therefore participate directly in chemical bonding. The number of valence electrons largely determines an element's reactivity and the types of chemical bonds it can form (ionic, covalent, metallic). Atoms tend to react in ways that achieve a stable electron configuration, often resembling the electron configuration of a noble gas (Group 18 elements). This drive towards stability is a fundamental principle in chemistry.
Electron Shells and Subshells: Organizing Electrons
Electrons are organized within atoms in specific energy levels called electron shells. Each shell can hold a maximum number of electrons, with the first shell (n=1) accommodating a maximum of two electrons, the second shell (n=2) up to eight, and so on. Within each shell, electrons are further organized into subshells, designated as s, p, d, and f. Each subshell can hold a specific number of electrons: s (2), p (6), d (10), and f (14).
This organized structure is fundamental to understanding electron configuration and ultimately, the number of valence electrons an atom possesses.
Sodium's Atomic Structure: Unveiling the Valence Electrons
Sodium (Na) has an atomic number of 11. This means a neutral sodium atom contains 11 protons and 11 electrons. To determine the number of valence electrons, we need to determine the electron configuration of sodium.
Electron Configuration of Sodium
The electron configuration of sodium is 1s²2s²2p⁶3s¹. Let's break this down:
- 1s²: Two electrons occupy the first shell's s subshell.
- 2s²: Two electrons occupy the second shell's s subshell.
- 2p⁶: Six electrons occupy the second shell's p subshell.
- 3s¹: One electron occupies the third shell's s subshell.
The outermost shell of sodium is the third shell (n=3), and it contains only one electron in the 3s subshell. Therefore, sodium has only one valence electron.
Visualizing Sodium's Electron Configuration
Imagine the sodium atom like a layered onion. The innermost layer (shell 1) holds two electrons, the next layer (shell 2) holds eight electrons, and the outermost layer (shell 3) contains only one solitary electron. This single electron is loosely held and readily participates in chemical reactions.
The Significance of Sodium's Single Valence Electron
The fact that sodium possesses only one valence electron has profound implications for its chemical behavior and properties. This single electron is easily lost, making sodium highly reactive. This reactivity is what makes sodium such a crucial element in various applications.
Sodium's Reactivity and Ionic Bonding
Sodium's strong tendency to lose its single valence electron leads to the formation of a stable sodium ion (Na⁺). By losing this electron, sodium achieves a stable octet (eight electrons) in its outermost shell, mimicking the electron configuration of neon, a noble gas. This electron loss is an example of ionization.
The lost electron is often gained by another atom, typically a non-metal with a high electronegativity (such as chlorine). This transfer of electrons results in the formation of an ionic bond, creating an ionic compound. A classic example is sodium chloride (NaCl), common table salt, formed by the ionic bond between Na⁺ and Cl⁻.
Metallic Bonding in Sodium
While sodium readily forms ionic bonds with non-metals, it also exhibits metallic bonding in its pure elemental form. Metallic bonding arises from the delocalization of valence electrons among a lattice of metal atoms. In sodium, the single valence electron becomes part of a "sea" of delocalized electrons, contributing to sodium's characteristic properties, such as high electrical and thermal conductivity and malleability.
Applications Leveraging Sodium's Reactivity
The unique properties stemming from sodium's single valence electron make it a crucial element in a wide range of applications:
- Sodium Chloride (NaCl): Table salt, a fundamental seasoning and food preservative, is a direct result of sodium's ionic bonding capabilities.
- Sodium Hydroxide (NaOH): Also known as lye or caustic soda, sodium hydroxide is used in various industrial processes, including soap making, paper production, and drain cleaning. Its strong alkaline nature is a direct result of sodium's reactivity.
- Sodium Lamps: Sodium vapor lamps, known for their bright yellow-orange light, utilize sodium's electronic transitions between energy levels to produce light efficiently.
- Organic Chemistry: Sodium's reactivity is exploited in numerous organic chemical reactions, acting as a reducing agent and playing a role in synthesis and other transformations.
Beyond Sodium: Valence Electrons Across the Periodic Table
Understanding sodium's single valence electron helps us understand the periodic trends in reactivity and bonding across the periodic table. Elements within the same group (vertical column) of the periodic table share the same number of valence electrons, exhibiting similar chemical behavior. For instance, lithium (Li), potassium (K), rubidium (Rb), and cesium (Cs), all in Group 1, also possess one valence electron and display similar reactivity to sodium.
Conclusion: The Importance of Understanding Valence Electrons
The number of valence electrons is a fundamental property that dictates an element's chemical behavior. Sodium, with its single valence electron, is a prime example of how this property governs reactivity, bonding, and ultimately, its wide range of applications. Understanding the electron configuration and the significance of valence electrons is essential not only for comprehending sodium's properties but also for grasping the broader principles of chemical bonding and reactivity across the entire periodic table. The simple yet crucial fact that sodium possesses one valence electron forms the foundation for understanding its vital role in our world, from the salt on our tables to industrial processes that shape modern life. Furthermore, this understanding extends to our comprehension of other elements and their interactions, solidifying the central role of valence electrons in the study of chemistry.
Latest Posts
Latest Posts
-
What Percentage Is 5 Of 30
Mar 29, 2025
-
How Many Kilometers In 10 Miles
Mar 29, 2025
-
How Big Is 70 Cm In Inches
Mar 29, 2025
-
6 Cups Is How Many Pints
Mar 29, 2025
-
How Many Liters Are In 500 Ml
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Much Valence Electrons Does Sodium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
