How To Calculate Molar Solubility From Ksp
Kalali
Mar 15, 2025 · 5 min read
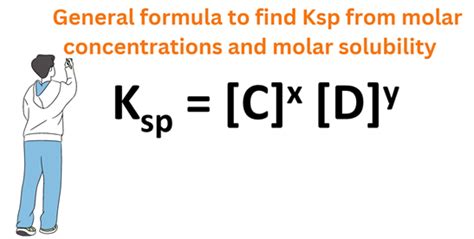
Table of Contents
How to Calculate Molar Solubility from Ksp: A Comprehensive Guide
The solubility product constant, Ksp, is a crucial concept in chemistry that quantifies the solubility of sparingly soluble ionic compounds. Understanding how to calculate molar solubility from Ksp is fundamental for various applications, including environmental chemistry, pharmaceutical science, and geochemistry. This comprehensive guide will walk you through the process, covering various scenarios and providing practical examples.
Understanding Ksp and Molar Solubility
Before diving into the calculations, let's clarify the definitions:
-
Solubility: This refers to the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure to form a saturated solution. We often express solubility in terms of molar solubility (S).
-
Molar Solubility (S): This represents the concentration of the dissolved solute (in moles per liter, mol/L or M) in a saturated solution. It's a direct measure of how much of the compound dissolves.
-
Solubility Product Constant (Ksp): This is an equilibrium constant that describes the extent to which a sparingly soluble ionic compound dissolves in water. It's the product of the concentrations of the ions raised to the powers of their stoichiometric coefficients in the balanced dissolution equation. A small Ksp value indicates low solubility, while a large Ksp value suggests higher solubility.
Calculating Molar Solubility from Ksp: Simple Cases
The simplest calculations involve compounds that dissociate into a 1:1 ratio of ions. Consider the general case of a sparingly soluble salt, MX:
MX(s) <=> M+(aq) + X-(aq)
The Ksp expression for this is:
Ksp = [M+][X-]
Since the molar solubility (S) is equal to the concentration of both M+ and X- ions in a saturated solution ([M+] = [X-] = S), we can rewrite the Ksp expression as:
Ksp = S²
Therefore, to find the molar solubility (S), simply take the square root of the Ksp value:
S = √Ksp
Example:
Let's say the Ksp of AgCl (silver chloride) is 1.8 x 10⁻¹⁰. To calculate its molar solubility:
S = √(1.8 x 10⁻¹⁰) ≈ 1.3 x 10⁻⁵ M
This means that at saturation, approximately 1.3 x 10⁻⁵ moles of AgCl dissolve per liter of water.
Calculating Molar Solubility from Ksp: More Complex Cases
More complex scenarios involve compounds that dissociate into more than two ions with different stoichiometric coefficients. Let's examine a few examples:
Case 1: Compounds with Different Stoichiometric Coefficients
Consider the dissolution of lead(II) iodide (PbI₂):
PbI₂(s) <=> Pb²+(aq) + 2I⁻(aq)
The Ksp expression is:
Ksp = [Pb²+][I⁻]²
If we let 'S' represent the molar solubility of PbI₂, then [Pb²+] = S and [I⁻] = 2S. Substituting these into the Ksp expression:
Ksp = S(2S)² = 4S³
Solving for S:
S = ³√(Ksp/4)
Case 2: Common Ion Effect
The common ion effect describes the decrease in solubility of a sparingly soluble salt when a soluble salt containing a common ion is added to the solution. Let's consider adding NaCl to a saturated AgCl solution. The presence of the common ion, Cl⁻, will shift the equilibrium to the left, decreasing the solubility of AgCl.
The Ksp expression remains the same:
Ksp = [Ag+][Cl-]
However, now we need to account for the additional Cl⁻ ions from NaCl. If the concentration of Cl⁻ from NaCl is [Cl⁻]₀, then the total concentration of Cl⁻ is [Cl⁻] = S + [Cl⁻]₀. Therefore:
Ksp = S(S + [Cl⁻]₀)
Solving this quadratic equation for S will give the molar solubility in the presence of the common ion.
Case 3: pH Effects on Solubility
The solubility of some salts is affected by pH. This is particularly true for salts of weak acids or bases. For example, consider the solubility of magnesium hydroxide, Mg(OH)₂:
Mg(OH)₂(s) <=> Mg²+(aq) + 2OH⁻(aq)
The hydroxide ion concentration is affected by the pH of the solution. A lower pH (more acidic) will reduce the hydroxide concentration, increasing the solubility of Mg(OH)₂. Conversely, a higher pH (more basic) will increase the hydroxide concentration, reducing solubility. To calculate molar solubility in this case, you need to incorporate the hydroxide ion concentration determined by the pH of the solution into the Ksp expression.
Advanced Considerations and Limitations
-
Activity vs. Concentration: The Ksp calculations presented here use concentrations. However, in solutions with high ionic strength, activities should be used instead of concentrations for more accurate results. Activity takes into account the interionic interactions that affect the effective concentration of ions.
-
Complex Ion Formation: The presence of complexing agents can significantly alter solubility. If the metal ion forms stable complexes, the solubility will increase. These complex formation reactions need to be incorporated into the equilibrium calculations.
-
Temperature Dependence: Ksp values are temperature-dependent. The calculations presented here are valid only at the temperature at which the Ksp value was determined.
-
Solid Phase Changes: In some cases, the solid phase may change, affecting solubility.
Practical Applications and Further Exploration
The ability to calculate molar solubility from Ksp is essential in many fields:
- Environmental Chemistry: Predicting the fate of pollutants in water systems.
- Geochemistry: Understanding mineral formation and dissolution in geological processes.
- Pharmaceutical Science: Formulating drugs with appropriate solubility characteristics.
- Analytical Chemistry: Developing and validating analytical methods.
This detailed guide provides a solid foundation for calculating molar solubility from Ksp. By understanding the different scenarios and considerations discussed above, you can tackle a wide range of solubility problems. Further exploration of advanced topics such as activity coefficients and complex ion formation will enhance your understanding and ability to accurately predict solubility in more complex systems. Remember to always consider the specific conditions and potential complicating factors relevant to your particular problem.
Latest Posts
Latest Posts
-
2 Meters Is How Many Cm
Mar 15, 2025
-
6 Out Of 14 As A Percentage
Mar 15, 2025
-
24 Of 40 Is What Percent
Mar 15, 2025
-
What Is 8 5 As A Percent
Mar 15, 2025
-
How Many Oz Is 1 1 3 Cup
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Molar Solubility From Ksp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
