How To Calculate The Ph At The Equivalence Point
Kalali
Mar 19, 2025 · 6 min read
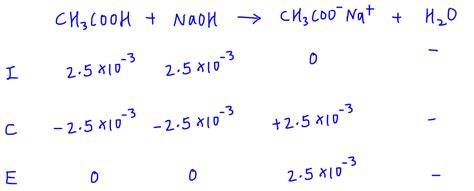
Table of Contents
How to Calculate the pH at the Equivalence Point of a Titration
Determining the pH at the equivalence point of a titration is crucial for understanding the reaction's completion and choosing appropriate indicators. The equivalence point signifies the stoichiometric point where the moles of acid and base are exactly equal, leading to neutralization. However, calculating the pH at this point can be tricky, as it depends heavily on the strength of the acid and base involved. This comprehensive guide will walk you through various scenarios, providing step-by-step calculations and explanations.
Understanding the Equivalence Point
Before diving into calculations, let's solidify our understanding of the equivalence point. In a titration, we gradually add a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until the reaction is complete. The equivalence point is reached when the moles of titrant added are chemically equivalent to the moles of analyte present.
Key considerations:
- Strong Acid-Strong Base Titrations: These titrations result in a neutral solution (pH 7) at the equivalence point because the resulting salt does not undergo hydrolysis.
- Weak Acid-Strong Base Titrations: The pH at the equivalence point will be greater than 7 (basic) due to the hydrolysis of the conjugate base of the weak acid.
- Strong Acid-Weak Base Titrations: The pH at the equivalence point will be less than 7 (acidic) due to the hydrolysis of the conjugate acid of the weak base.
- Weak Acid-Weak Base Titrations: These titrations are more complex, and the pH at the equivalence point is determined by the relative strengths of the acid and base.
Calculating pH at the Equivalence Point: Different Scenarios
1. Strong Acid-Strong Base Titration
This is the simplest scenario. The pH at the equivalence point is 7.0 (assuming 25°C). No further calculations are needed. The neutralization reaction completely consumes both the acid and base, leaving only water and a neutral salt.
Example: Titration of 0.1 M HCl with 0.1 M NaOH. At the equivalence point, the solution is simply NaCl and water, resulting in a neutral pH of 7.
2. Weak Acid-Strong Base Titration
This is more complex because the conjugate base of the weak acid hydrolyzes, affecting the pH. Here's how to calculate the pH:
-
Determine the concentration of the conjugate base: At the equivalence point, all the weak acid (HA) has been converted to its conjugate base (A⁻). The concentration of A⁻ can be calculated using the initial moles of HA and the total volume of the solution at the equivalence point.
-
Use the Kb expression: The conjugate base will react with water according to the following equilibrium: A⁻ + H₂O ⇌ HA + OH⁻
The Kb expression is: Kb = ([HA][OH⁻])/[A⁻]
Kb can be calculated from the Ka of the weak acid using the relationship: Kw = Ka * Kb (where Kw is the ion product of water, 1.0 x 10⁻¹⁴ at 25°C)
-
Solve for [OH⁻]: Using an ICE table (Initial, Change, Equilibrium) and the Kb value, solve for [OH⁻]. Often, simplifying assumptions can be made (x is small compared to the initial concentration).
-
Calculate pOH: pOH = -log[OH⁻]
-
Calculate pH: pH = 14 - pOH
Example: Titration of 0.1 M acetic acid (Ka = 1.8 x 10⁻⁵) with 0.1 M NaOH. Assume 25 mL of acetic acid is titrated to equivalence.
-
1. Concentration of conjugate base (acetate): If 25 mL of 0.1 M acetic acid is neutralized, this means 2.5 mmol of acetate is formed. Assuming that the volume doubles, the concentration of acetate is approximately 0.05 M.
-
2. Kb calculation: Kb = Kw/Ka = (1.0 x 10⁻¹⁴)/(1.8 x 10⁻⁵) ≈ 5.6 x 10⁻¹⁰
-
3. Solving for [OH⁻]: Using an ICE table and the Kb expression, we solve for [OH⁻]. The simplified calculation (assuming x is small) gives a reasonably accurate approximation.
-
4. & 5. pOH and pH calculations: Once [OH⁻] is known, we calculate pOH and subsequently pH.
3. Strong Acid-Weak Base Titration
This is analogous to the weak acid-strong base titration, but with the conjugate acid hydrolyzing.
-
Determine the concentration of the conjugate acid: At the equivalence point, all the weak base (B) has been converted to its conjugate acid (BH⁺).
-
Use the Ka expression: The conjugate acid will react with water: BH⁺ + H₂O ⇌ B + H₃O⁺
The Ka expression is: Ka = ([B][H₃O⁺])/[BH⁺]
Ka can be calculated from the Kb of the weak base using the relationship: Kw = Ka * Kb
-
Solve for [H₃O⁺]: Using an ICE table and the Ka value, solve for [H₃O⁺].
-
Calculate pH: pH = -log[H₃O⁺]
Example: Titration of 0.1 M ammonia (Kb = 1.8 x 10⁻⁵) with 0.1 M HCl. Similar steps as the weak acid-strong base titration are followed, but using the Ka expression and solving for [H₃O⁺] instead of [OH⁻].
4. Weak Acid-Weak Base Titration
This is the most complex scenario because both the conjugate acid and conjugate base can hydrolyze. The pH at the equivalence point is determined by the relative strengths of the acid and base. A simple approximation is often insufficient, and more sophisticated methods like the Henderson-Hasselbalch equation may be employed (however, it should be applied cautiously at the equivalence point as it's designed for buffer solutions, not equivalence points). Often, it's necessary to use more advanced equilibrium calculations involving the simultaneous consideration of both hydrolysis reactions. The exact calculations are best approached using a numerical solver. The pH will be approximately 7 if the Ka and Kb are equal; otherwise, it will deviate towards the acidic or basic side, depending on the relative strengths.
Practical Considerations and Approximations
-
Simplifying assumptions: In many cases, simplifying assumptions can be made (like ignoring the contribution of water's autoionization or assuming x is negligible compared to initial concentrations) to simplify the calculations. However, it's crucial to check the validity of these assumptions after the calculation to ensure accuracy.
-
Iterative methods: For more precise calculations, especially in complex scenarios, iterative methods may be necessary. These methods involve repeatedly refining the solution until a desired level of accuracy is reached.
-
Software and calculators: Numerous online calculators and software packages are available to assist in these calculations, reducing the risk of errors in complex situations.
-
Temperature dependence: The Kw value is temperature-dependent. The calculations presented here assume a temperature of 25°C. At other temperatures, the Kw value must be adjusted accordingly.
Choosing the Right Indicator
The pH at the equivalence point is critical for selecting a suitable indicator for the titration. The indicator's color change should occur within the pH range close to the equivalence point to ensure accurate determination of the endpoint. The ideal indicator's pKa should be close to the expected pH at the equivalence point.
Conclusion
Calculating the pH at the equivalence point of a titration can range from straightforward (strong acid-strong base) to complex (weak acid-weak base). Understanding the concepts of acid-base equilibrium, hydrolysis, and the relevant expressions is essential. While simplifying assumptions are often used to facilitate calculations, it's vital to verify their validity. For more accurate results in complex scenarios, iterative methods or numerical solvers are recommended. Remember, selecting the appropriate indicator is also crucial for accurate experimental determination of the equivalence point. This comprehensive guide provides a solid foundation for understanding and tackling these calculations, enabling you to accurately analyze acid-base titrations.
Latest Posts
Latest Posts
-
How Many Millimeters Is 8 In
Mar 19, 2025
-
The Movement Of One Object Around Another
Mar 19, 2025
-
What Do Surge Protectors Help Prevent In The Workplace
Mar 19, 2025
-
How Does Dna In Cells Determine An Organisms Complex Traits
Mar 19, 2025
-
Can A Polygon Have A Curved Side
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
