How To Find Kb From Ph
Kalali
Mar 18, 2025 · 6 min read
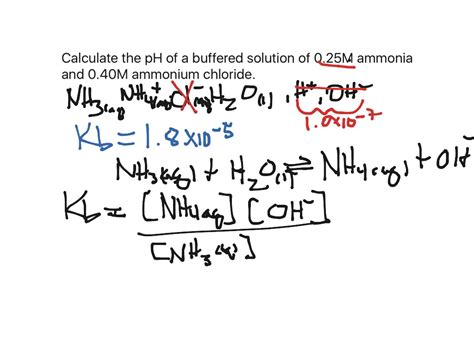
Table of Contents
How to Find Kb from Ph: A Comprehensive Guide
Determining the Kb (base dissociation constant) from the pH of a solution might seem daunting at first, but with a systematic approach and a solid understanding of the underlying chemistry, it becomes manageable. This comprehensive guide will walk you through various methods, detailing the calculations and considerations involved. We'll cover scenarios involving weak bases, strong bases, and the implications of buffer solutions. By the end, you'll be equipped to confidently calculate Kb from pH data.
Understanding the Fundamentals: pH, pOH, Kw, and Kb
Before diving into the calculations, let's review the essential concepts:
-
pH: The negative logarithm of the hydrogen ion concentration ([H⁺]), representing the acidity of a solution. A lower pH indicates higher acidity.
-
pOH: The negative logarithm of the hydroxide ion concentration ([OH⁻]), representing the basicity of a solution. A lower pOH indicates higher basicity.
-
Kw (Ionic Product of Water): At 25°C, Kw = [H⁺][OH⁻] = 1.0 x 10⁻¹⁴. This constant relationship between [H⁺] and [OH⁻] is crucial for converting between pH and pOH. The equation is: pH + pOH = 14 at 25°C.
-
Kb (Base Dissociation Constant): This equilibrium constant represents the extent to which a weak base dissociates in water. A larger Kb value signifies a stronger base. For a generic weak base, B, the dissociation reaction and Kb expression are:
B + H₂O ⇌ BH⁺ + OH⁻
Kb = [BH⁺][OH⁻] / [B]
Method 1: Calculating Kb for a Weak Base from its pH
This method is applicable when you know the initial concentration of the weak base and the pH of its solution. Here's a step-by-step approach:
-
Determine the pOH: Use the relationship pH + pOH = 14 to find the pOH of the solution.
-
Calculate the [OH⁻]: Use the definition of pOH: [OH⁻] = 10⁻ᵖᵒʰ
-
Construct an ICE table: An ICE (Initial, Change, Equilibrium) table helps organize the equilibrium concentrations. Consider the generic weak base dissociation:
B BH⁺ OH⁻ Initial Cᵢ 0 0 Change -x +x +x Equil. Cᵢ - x x x Where Cᵢ is the initial concentration of the weak base.
-
Substitute into the Kb expression: Substitute the equilibrium concentrations from the ICE table into the Kb expression:
Kb = x² / (Cᵢ - x)
-
Solve for x: In many cases, you can approximate Cᵢ - x ≈ Cᵢ, simplifying the equation to Kb ≈ x²/Cᵢ. This approximation is valid when x << Cᵢ (typically when Kb is small). If the approximation is not valid, you'll need to solve the quadratic equation.
-
Calculate Kb: Substitute the value of x ([OH⁻]) into the Kb expression to determine the Kb value.
Example:
A 0.10 M solution of a weak base has a pH of 9.0. Calculate the Kb.
-
pOH = 14 - pH = 14 - 9.0 = 5.0
-
[OH⁻] = 10⁻⁵⁰ = 1.0 x 10⁻⁵ M
-
ICE table (using the approximation Cᵢ - x ≈ Cᵢ):
B BH⁺ OH⁻ Initial 0.10 M 0 0 Change -x +x +x Equil. 0.10 M x x -
Kb ≈ x²/Cᵢ = (1.0 x 10⁻⁵)² / 0.10 = 1.0 x 10⁻⁹
Therefore, the Kb of the weak base is approximately 1.0 x 10⁻⁹.
Method 2: Calculating Kb from the pKa of the Conjugate Acid
This method leverages the relationship between Ka (acid dissociation constant) and Kb:
Kw = Ka * Kb
If you know the pKa of the conjugate acid (BH⁺) of the weak base, you can calculate Ka and then use the above equation to find Kb.
-
Calculate Ka: Ka = 10⁻ᵖᵏᵃ
-
Calculate Kb: Kb = Kw / Ka = 1.0 x 10⁻¹⁴ / Ka
Example:
The pKa of the conjugate acid of a weak base is 5.0. Calculate the Kb.
-
Ka = 10⁻⁵⁰ = 1.0 x 10⁻⁵
-
Kb = 1.0 x 10⁻¹⁴ / 1.0 x 10⁻⁵ = 1.0 x 10⁻⁹
Method 3: Dealing with Strong Bases
Strong bases completely dissociate in water, making the calculation simpler. You don't need an ICE table or Kb.
-
Find [OH⁻]: From the pOH (calculated from pH), find [OH⁻] = 10⁻ᵖᵒʰ. Since it's a strong base, this concentration is directly related to the initial concentration of the base.
-
Determine Kb: The Kb value for strong bases is generally very large and not typically used in calculations as they are considered to fully dissociate.
Method 4: Considerations for Buffer Solutions
Buffer solutions contain both a weak acid and its conjugate base (or a weak base and its conjugate acid), resisting changes in pH upon addition of small amounts of acid or base. Calculating Kb from the pH of a buffer requires the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
Where [A⁻] is the concentration of the conjugate base and [HA] is the concentration of the weak acid. This method is applicable if you know the concentrations of the acid and its conjugate base within the buffer solution and the pH of the buffer. Remember to use the relationship between Ka and Kb to calculate Kb from the given pKa.
Advanced Considerations and Limitations
-
Temperature Dependence: Kw, Ka, and Kb are temperature-dependent. The values provided (Kw = 1.0 x 10⁻¹⁴) are valid at 25°C. At different temperatures, these values will change, affecting the calculated Kb.
-
Ionic Strength: High ionic strength in the solution can affect the activity coefficients of the ions, altering the equilibrium and the calculated Kb. Activity corrections may be necessary for precise calculations in high-ionic-strength solutions.
-
Approximation Limitations: The approximation used in Method 1 (Cᵢ - x ≈ Cᵢ) is only valid when the base is weak (small Kb) and the initial concentration is relatively high. If the approximation is not valid, you need to solve a quadratic equation.
-
Polyprotic Bases: For bases that can accept more than one proton (polyprotic bases), you'll have multiple Kb values, each corresponding to a different dissociation step.
Conclusion:
Calculating Kb from pH involves a multi-step process that depends on the type of base (weak or strong) and the context (buffer solution or not). While the fundamental principles remain the same, careful attention to detail, including the assumptions made and potential limitations, is critical for accurate results. Mastering these methods empowers you to understand and quantify the basicity of solutions, a fundamental skill in chemistry and related fields. Remember to always check your assumptions and consider potential sources of error for a more comprehensive understanding of your results.
Latest Posts
Latest Posts
-
Is Sour Taste A Physical Property
Mar 19, 2025
-
How Many Kilos Are 20 Pounds
Mar 19, 2025
-
11 Out Of 30 As A Percentage
Mar 19, 2025
-
Cuanto Es 92 Grados Fahrenheit En Centigrados
Mar 19, 2025
-
How To Get Magnitude Of Force
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Find Kb From Ph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
