How To Find The Change In Enthalpy
Kalali
Mar 19, 2025 · 7 min read
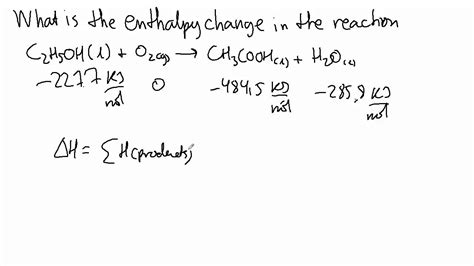
Table of Contents
How to Find the Change in Enthalpy: A Comprehensive Guide
Enthalpy, denoted by H, is a crucial thermodynamic property representing the total heat content of a system at constant pressure. The change in enthalpy (ΔH) signifies the heat absorbed or released during a process at constant pressure. Understanding how to find ΔH is essential in various fields, from chemistry and physics to engineering and environmental science. This comprehensive guide will explore various methods for determining the change in enthalpy, focusing on different scenarios and applications.
Understanding Enthalpy and its Change
Before delving into the methods, let's solidify our understanding of enthalpy and its change. Enthalpy itself isn't directly measurable; we can only measure the change in enthalpy. This change is particularly relevant in chemical reactions and physical processes.
- Exothermic Reactions: In exothermic reactions, heat is released to the surroundings, resulting in a negative ΔH (ΔH < 0). The system's enthalpy decreases.
- Endothermic Reactions: Conversely, in endothermic reactions, heat is absorbed from the surroundings, leading to a positive ΔH (ΔH > 0). The system's enthalpy increases.
The magnitude of ΔH indicates the amount of heat transferred during the process. A larger ΔH signifies a greater heat transfer.
Methods for Determining the Change in Enthalpy (ΔH)
Several methods exist for calculating ΔH, each suited for specific situations:
1. Using Calorimetry
Calorimetry is a direct experimental method for measuring the heat transfer during a process. A calorimeter, a device designed to isolate the system from its surroundings, measures the temperature change caused by the reaction. This temperature change, along with the heat capacity of the calorimeter and the amount of substance, allows us to calculate ΔH.
Types of Calorimetry:
-
Constant-pressure calorimetry (coffee-cup calorimeter): This simple method is suitable for reactions at atmospheric pressure. The heat absorbed or released by the reaction is directly related to the temperature change of the solution. The equation used is:
ΔH = -q<sub>solution</sub> / n
where:
- ΔH is the change in enthalpy per mole of reactant.
- q<sub>solution</sub> is the heat absorbed or released by the solution (q<sub>solution</sub> = m<sub>solution</sub> * C<sub>solution</sub> * ΔT), where m is the mass, C is the specific heat capacity, and ΔT is the temperature change.
- n is the number of moles of the limiting reactant.
-
Constant-volume calorimetry (bomb calorimeter): This method is used for reactions involving gases, where volume changes are significant. The heat released by the reaction is transferred to the surrounding water, and the temperature increase is measured. Corrections may need to be applied to account for the heat absorbed by the calorimeter itself.
Limitations of Calorimetry: Calorimetry requires careful experimental design and precise measurements. Heat loss to the surroundings can lead to errors in the ΔH calculation. The method is also not suitable for all types of reactions.
2. Using Hess's Law
Hess's Law states that the total enthalpy change for a reaction is independent of the pathway taken. This means that the overall ΔH for a reaction can be calculated by summing the ΔH values of a series of intermediate reactions that add up to the overall reaction. This method is particularly useful when the direct measurement of ΔH is difficult or impossible.
Applying Hess's Law:
- Write the target reaction: Clearly define the overall reaction for which you want to determine ΔH.
- Find intermediate reactions: Identify a series of reactions with known ΔH values that, when added together, yield the target reaction. You might need to reverse some reactions or multiply them by a factor to ensure that the reactants and products cancel appropriately.
- Manipulate ΔH values: If a reaction is reversed, the sign of its ΔH is changed. If a reaction is multiplied by a factor, its ΔH is multiplied by the same factor.
- Sum the ΔH values: Add the adjusted ΔH values for the intermediate reactions to obtain the overall ΔH for the target reaction.
Example: Let's say we want to find ΔH for the reaction A + B → C, but we only have the ΔH values for A → D (ΔH1) and D + B → C (ΔH2). We can use Hess's Law to calculate ΔH for the target reaction: ΔH = ΔH1 + ΔH2.
3. Using Standard Enthalpies of Formation (ΔH<sub>f</sub>°)
The standard enthalpy of formation (ΔH<sub>f</sub>°) is the change in enthalpy when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm). These values are tabulated for many compounds. We can use these standard enthalpies of formation to calculate the ΔH for a reaction using the following equation:
ΔH°<sub>rxn</sub> = Σ [ΔH<sub>f</sub>°(products)] - Σ [ΔH<sub>f</sub>°(reactants)]
where:
- ΔH°<sub>rxn</sub> is the standard enthalpy change for the reaction.
- Σ represents the sum.
- ΔH<sub>f</sub>°(products) are the standard enthalpies of formation of the products.
- ΔH<sub>f</sub>°(reactants) are the standard enthalpies of formation of the reactants.
Important Considerations: Remember that the standard enthalpy of formation of an element in its standard state is zero. This equation calculates the standard enthalpy change, meaning it applies to reactions conducted under standard conditions.
4. Using Bond Energies
Bond energy is the amount of energy required to break one mole of a specific type of bond in a gaseous molecule. These values are also tabulated. We can estimate the enthalpy change of a reaction using bond energies by considering the bonds broken and formed during the reaction.
Calculating ΔH using bond energies:
ΔH ≈ Σ (bond energies of bonds broken) - Σ (bond energies of bonds formed)
Limitations of this method: This method provides an estimate of ΔH, as it ignores factors like intermolecular forces and the effects of the phase of the reactants and products. The accuracy of the estimate depends on the availability and accuracy of the bond energy data.
5. Using Kirchhoff's Law
Kirchhoff's Law relates the change in enthalpy at one temperature to the change in enthalpy at another temperature. It's particularly useful when you know ΔH at one temperature but need to determine it at a different temperature. The equation is:
ΔH<sub>T2</sub> = ΔH<sub>T1</sub> + ∫<sub>T1</sub><sup>T2</sup> ΔC<sub>p</sub> dT
where:
- ΔH<sub>T1</sub> is the change in enthalpy at temperature T1.
- ΔH<sub>T2</sub> is the change in enthalpy at temperature T2.
- ΔC<sub>p</sub> is the change in heat capacity at constant pressure between products and reactants. This is usually assumed to be constant over a small temperature range.
This integration can be simplified if ΔC<sub>p</sub> is assumed to be constant:
ΔH<sub>T2</sub> ≈ ΔH<sub>T1</sub> + ΔC<sub>p</sub>(T2 - T1)
Important Note: This equation assumes that ΔC<sub>p</sub> is relatively constant over the temperature range. For larger temperature differences, a more accurate calculation may require considering the temperature dependence of ΔC<sub>p</sub>.
Choosing the Appropriate Method
The best method for determining ΔH depends on the specific circumstances:
- Calorimetry: Ideal for direct experimental measurement, suitable for reactions where temperature change is easily measurable.
- Hess's Law: Useful when direct measurement is difficult, relies on available data for intermediate reactions.
- Standard Enthalpies of Formation: Convenient if standard enthalpy of formation data is available for all reactants and products.
- Bond Energies: Provides an estimate, particularly helpful when other data is unavailable.
- Kirchhoff's Law: Necessary for correcting ΔH values to different temperatures.
By understanding these different methods and their limitations, you can effectively determine the change in enthalpy for a wide range of chemical and physical processes. Remember to always carefully consider the assumptions and limitations of each method to ensure the accuracy and reliability of your results. Accurate calculation of ΔH is crucial in many scientific and engineering applications, allowing for better prediction of reaction feasibility and energy balance calculations.
Latest Posts
Latest Posts
-
24 Out Of 32 As A Percentage
May 09, 2025
-
What Are All The Factors Of 92
May 09, 2025
-
Coal Is Formed In Which Of The Following Depositional Environments
May 09, 2025
-
How Do You Calculate Magnitude Of Force
May 09, 2025
-
26 To The Power Of 2
May 09, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Change In Enthalpy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.