How To Find The Heat Of Solution
Kalali
Mar 26, 2025 · 6 min read
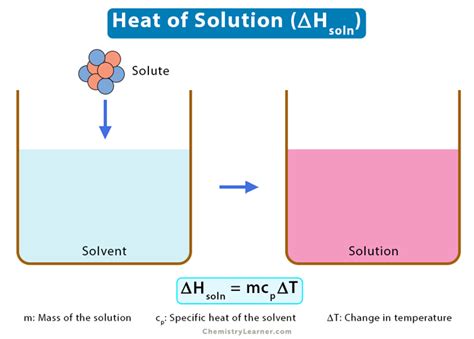
Table of Contents
How to Find the Heat of Solution: A Comprehensive Guide
Determining the heat of solution, also known as the enthalpy of dissolution, is a crucial aspect of chemistry and numerous industrial processes. Understanding this thermodynamic property allows us to predict the behavior of solutions, design efficient reactions, and optimize various applications. This comprehensive guide delves into the methods and calculations involved in finding the heat of solution, equipping you with the knowledge to tackle this important concept.
What is the Heat of Solution?
The heat of solution (ΔH<sub>soln</sub>) refers to the change in enthalpy that occurs when a specified amount of solute dissolves in a specified amount of solvent at constant pressure. This process can be either exothermic, releasing heat into the surroundings (ΔH<sub>soln</sub> < 0), or endothermic, absorbing heat from the surroundings (ΔH<sub>soln</sub> > 0). The magnitude of the heat of solution depends on several factors, including the nature of the solute and solvent, their concentrations, and the temperature.
Factors Affecting the Heat of Solution
Several factors intricately influence the heat of solution. Understanding these is critical for accurate predictions and experimental design.
1. Solute-Solvent Interactions:
The dominant factor is the interplay between solute-solute, solvent-solvent, and solute-solvent interactions. If the solute-solvent interactions are significantly stronger than the solute-solute and solvent-solvent interactions, the dissolution process is typically exothermic. Conversely, if the solute-solvent interactions are weaker, the process tends to be endothermic.
2. Nature of the Solute and Solvent:
The chemical nature of both solute and solvent plays a significant role. Polar solutes tend to dissolve readily in polar solvents (like water), while nonpolar solutes dissolve better in nonpolar solvents (like hexane). This is governed by the principle of "like dissolves like". Ionic compounds, for instance, often exhibit significant heat of solution due to the strong ion-dipole interactions with polar solvents.
3. Concentration:
The heat of solution is typically reported for a specific concentration, often expressed as molarity or molality. The heat of solution can vary with concentration, especially at higher concentrations where solute-solute interactions become increasingly important.
4. Temperature:
Temperature influences the kinetic energy of molecules, affecting the rate of dissolution and, to a lesser extent, the enthalpy change. While the overall heat of solution may not change dramatically with small temperature variations, the rate at which the process occurs can be significantly affected.
Methods for Determining the Heat of Solution
Several experimental techniques can be employed to determine the heat of solution. The choice of method depends largely on the precision required and the nature of the solute and solvent.
1. Calorimetry:
Calorimetry is the most common method for measuring the heat of solution. This technique involves measuring the temperature change that occurs when a solute dissolves in a solvent within an insulated container called a calorimeter. The heat absorbed or released by the solution is directly related to the temperature change and the heat capacity of the calorimeter and its contents.
Types of Calorimeters:
- Coffee-cup calorimeter: This simple, inexpensive calorimeter uses a styrofoam cup to minimize heat exchange with the surroundings. It's suitable for approximate measurements.
- Bomb calorimeter: A more sophisticated type, used for precise measurements of heat changes, especially for combustion reactions.
- Differential scanning calorimeter (DSC): This instrument precisely measures the heat flow associated with physical and chemical transformations, including dissolution.
Calculations:
The fundamental equation used in calorimetry is:
q = mcΔT
Where:
- q is the heat absorbed or released (in Joules)
- m is the mass of the solution (in grams)
- c is the specific heat capacity of the solution (usually approximated as the specific heat capacity of the solvent, in J/g°C)
- ΔT is the change in temperature (in °C)
The heat of solution (ΔH<sub>soln</sub>) is then calculated by dividing the heat (q) by the number of moles of solute dissolved:
ΔH<sub>soln</sub> = q / n
Where 'n' is the number of moles of solute.
2. Hess's Law:
For reactions where direct calorimetric measurement is difficult, Hess's Law provides an indirect approach. This law states that the total enthalpy change for a reaction is independent of the pathway taken. By combining the enthalpy changes of other reactions, the heat of solution can be calculated. This method involves using known enthalpy changes for related reactions, such as lattice energy and hydration energy for ionic compounds.
3. Computational Methods:
Advanced computational methods, like molecular dynamics and density functional theory (DFT), can predict the heat of solution. These techniques are particularly useful for complex systems and situations where experimental measurements are challenging. While computationally intensive, they offer valuable insights into the underlying molecular interactions driving the dissolution process.
Practical Considerations and Error Analysis
Several practical aspects are crucial for accurate results when determining the heat of solution.
- Accuracy of Temperature Measurement: Precise temperature measurements are vital. Using calibrated thermometers or thermocouples is recommended.
- Heat Loss: Minimize heat exchange with the surroundings by using well-insulated calorimeters and performing experiments quickly.
- Complete Dissolution: Ensure the solute completely dissolves before recording the final temperature. Stirring the solution helps achieve this.
- Solvent Purity: Using pure solvents is essential to avoid unexpected interactions that may affect the heat of solution.
- Uncertainty Analysis: Conducting multiple trials and performing a proper uncertainty analysis are important for evaluating the reliability of the results. Errors can arise from temperature measurements, heat capacity estimations, and weighing uncertainties. Propagation of error techniques should be employed to estimate the overall uncertainty in the final ΔH<sub>soln</sub> value.
Applications of Heat of Solution
The heat of solution has broad applications across various fields:
- Chemical Engineering: Designing efficient chemical processes, predicting solubility, and optimizing reaction conditions.
- Pharmaceutical Industry: Formulating drugs, understanding drug dissolution rates, and predicting bioavailability.
- Environmental Science: Assessing the environmental impact of dissolving substances, modeling pollutant behavior in water systems.
- Materials Science: Developing new materials with desired dissolution properties, designing controlled release systems.
- Geochemistry: Understanding mineral solubility in geological processes, modeling geochemical reactions.
Conclusion
Determining the heat of solution is a critical aspect of chemistry and numerous related fields. Understanding the underlying principles, mastering the experimental techniques (primarily calorimetry), and carefully considering the factors affecting the heat of solution are vital for accurate and reliable measurements. This comprehensive guide provides a solid foundation for tackling this important concept, whether you're a student, researcher, or professional working in a relevant field. Remember to always prioritize safety when conducting experiments and to meticulously record data for accurate analysis and reporting. The ability to accurately determine and interpret the heat of solution provides invaluable insights into the thermodynamic behavior of solutions and enables informed decisions in various applications.
Latest Posts
Latest Posts
-
How Many Minutes Is In 5 Hours
Mar 29, 2025
-
Is Chicken Noodle Soup A Heterogeneous Mixture
Mar 29, 2025
-
How Many Cups Is 5 Fl Oz
Mar 29, 2025
-
What Percentage Is 5 Of 30
Mar 29, 2025
-
How Many Kilometers In 10 Miles
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Heat Of Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
