How To Separate Water From Sugar
Kalali
Mar 15, 2025 · 6 min read
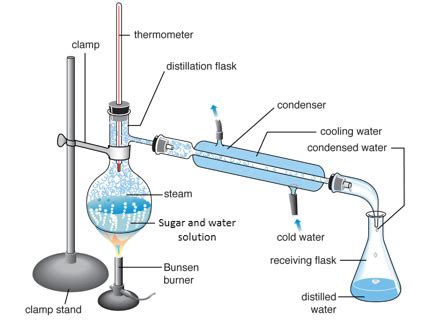
Table of Contents
How to Separate Water from Sugar: A Comprehensive Guide
Separating water from sugar is a fundamental chemistry concept with various applications, from everyday kitchen tasks to industrial processes. Understanding the principles behind this separation is crucial for effectively tackling different scenarios. This comprehensive guide delves into the various methods available, explaining their principles, advantages, and disadvantages. We'll explore everything from simple evaporation to more advanced techniques, equipping you with the knowledge to choose the most suitable method for your needs.
Understanding the Mixture: Water and Sugar
Before we delve into the separation techniques, it's important to understand the nature of the mixture. When sugar (sucrose) is dissolved in water, it forms a homogeneous solution. This means the sugar molecules are evenly distributed throughout the water, creating a single, uniform phase. There's no visible separation between the sugar and the water. This characteristic significantly impacts the methods we can use to separate them. The key to successful separation lies in exploiting the different physical properties of water and sugar, such as their boiling points and vapor pressures.
Methods for Separating Water from Sugar
Several methods can effectively separate water from sugar. The choice of method depends on factors like the scale of the separation (laboratory vs. industrial), desired purity of the water, and available resources. Let's explore some of the most common and effective techniques:
1. Evaporation
Principle: Evaporation leverages the difference in boiling points between water (100°C at standard atmospheric pressure) and sugar (decomposes before reaching its boiling point). By heating the solution, the water evaporates, leaving behind the sugar.
Process:
- Heat the Sugar Solution: Gently heat the sugar solution in a suitable container, such as a beaker or saucepan. A double boiler is ideal to prevent scorching.
- Water Evaporation: As the water heats, it will evaporate and turn into water vapor.
- Sugar Crystallization: As the water evaporates, the concentration of sugar increases. Eventually, the sugar will reach saturation and begin to crystallize, forming solid sugar at the bottom of the container.
- Collect the Sugar: Once all the water has evaporated, you'll be left with solid sugar crystals.
Advantages:
- Simplicity: This is a straightforward method, requiring minimal equipment.
- Cost-effectiveness: It's inexpensive, utilizing readily available heat sources.
Disadvantages:
- Time-consuming: Evaporation can be a slow process, especially for large volumes of solution.
- Potential for Sugar Degradation: Excessive heat can caramelize the sugar, altering its properties and potentially producing unwanted byproducts.
- Impurities: If impurities are present in the initial sugar solution, they will remain with the sugar crystals after evaporation.
2. Distillation
Principle: Distillation, a more refined technique than evaporation, utilizes the difference in boiling points to separate the components of a liquid mixture. It involves boiling the solution and then condensing the vapor to collect the purified liquid (in this case, water).
Process:
- Boiling the Solution: Heat the sugar solution in a distillation flask. The water will boil and turn into steam.
- Condensation: The steam rises and enters a condenser, where it cools and condenses back into liquid water.
- Collection: The purified water is collected in a separate container. The sugar remains in the distillation flask.
Advantages:
- Higher Purity: Distillation provides higher purity water compared to simple evaporation.
- Suitable for Large-Scale Operations: Distillation can be scaled up for industrial applications.
Disadvantages:
- More Complex Setup: Distillation requires specialized equipment like a distillation flask, condenser, and collection flask.
- Energy Intensive: The process requires significant energy input for heating and cooling.
- Sugar Loss: Although minimal, some sugar molecules might be carried over with the water vapor during distillation, impacting the purity of the collected water.
3. Reverse Osmosis
Principle: Reverse osmosis uses a semipermeable membrane to separate water from dissolved substances, including sugar. This membrane allows water molecules to pass through but blocks larger sugar molecules. Pressure is applied to force the water through the membrane, leaving behind the sugar.
Process:
- Application of Pressure: The sugar solution is pressurized and passed through a semipermeable membrane.
- Water Filtration: Water molecules pass through the membrane, while the sugar molecules are retained.
- Water Collection: The purified water is collected on the other side of the membrane.
Advantages:
- High Purity Water: Reverse osmosis produces highly purified water, free from most dissolved solids.
- Effective for Various Impurities: It's effective at removing not only sugar but also other dissolved substances.
Disadvantages:
- High Initial Cost: The equipment for reverse osmosis is relatively expensive.
- Membrane Maintenance: The membranes require regular maintenance and replacement.
- Energy Consumption: Applying pressure requires energy, increasing the operating cost.
4. Chromatography
Principle: Chromatography separates substances based on their differing affinities for a stationary and mobile phase. In the context of separating water and sugar, we can utilize different types of chromatography, though it's less practical than other methods for this specific separation.
Advantages:
- High Resolution: Chromatography offers high resolution separation of complex mixtures, but less effective for simple sugar/water mixture.
Disadvantages:
- Complexity: Chromatographic techniques are typically more complex and require specialized equipment and expertise.
- Not Ideal for Large-Scale Separation: Not practical for separating large volumes of sugar water.
Choosing the Right Method
The most suitable method for separating water from sugar depends on your specific needs:
- Small-scale separation with readily available equipment? Evaporation is the simplest and most cost-effective option.
- Need high-purity water? Distillation or reverse osmosis are preferable.
- Large-scale industrial separation? Distillation or reverse osmosis, with their scalability, are more appropriate.
- Dealing with a complex mixture beyond just sugar and water? Chromatography may be necessary, though for simple sugar and water, it is inefficient.
Safety Precautions
Regardless of the method chosen, safety precautions are paramount:
- Heat Safety: When using heat sources, ensure proper ventilation and avoid direct contact with hot surfaces.
- Glassware Handling: If using glassware, handle it carefully to avoid breakage.
- Electrical Safety: If using electrical equipment, ensure proper grounding and avoid contact with water.
Conclusion
Separating water from sugar involves leveraging the differing physical properties of these two substances. While evaporation offers a simple and accessible solution, distillation and reverse osmosis provide higher purity water, albeit with increased complexity and cost. Understanding the principles behind each method empowers you to choose the most appropriate technique for your specific requirements. By following the guidelines outlined in this guide, and prioritizing safety, you can efficiently and effectively separate water from sugar in various contexts. Remember to always adapt your approach to the scale and complexity of your separation task. This knowledge is not only valuable for kitchen experiments but also applicable to numerous industrial and scientific applications.
Latest Posts
Latest Posts
-
How Many Combinations Of Phone Numbers Are There
Mar 15, 2025
-
Common Multiple Of 3 4 5
Mar 15, 2025
-
What Is 112 F In Celsius
Mar 15, 2025
-
Cuanto Es 35 Pulgadas En Metros
Mar 15, 2025
-
What Is The Percent Of 1 50
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How To Separate Water From Sugar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
