In A Double Covalent Bond A Carbon Atom Shares
Kalali
Mar 13, 2025 · 6 min read
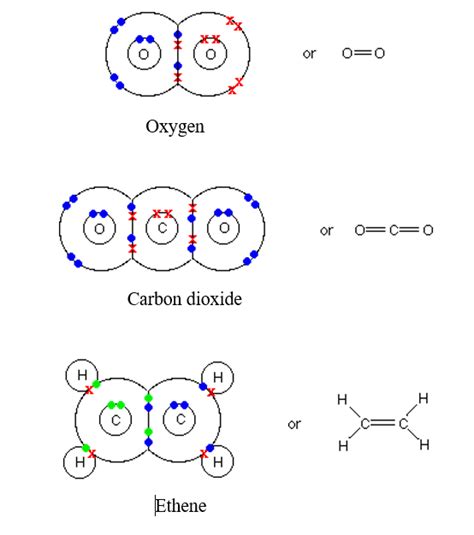
Table of Contents
In a Double Covalent Bond, a Carbon Atom Shares... Two Pairs of Electrons! Unveiling the Secrets of Carbon's Bonding Prowess
Carbon, the cornerstone of organic chemistry and the building block of life, possesses a unique ability to form a diverse array of molecules. This versatility stems primarily from its capacity to form covalent bonds, particularly double covalent bonds. Understanding how carbon shares electrons in these double bonds is crucial to grasping the fundamental principles of organic chemistry and the complexity of life itself. This article delves deep into the intricacies of carbon's double covalent bonding, exploring its implications for molecular structure, reactivity, and the properties of countless organic compounds.
The Fundamentals of Covalent Bonding
Before delving into the specifics of double bonds, let's briefly review the concept of covalent bonding. Covalent bonds arise from the sharing of electron pairs between atoms. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (a full outer electron shell). By sharing electrons, atoms effectively "complete" their outermost electron shells, gaining stability. The strength of a covalent bond depends on the degree of overlap between the atomic orbitals involved in the sharing process.
Carbon, with four electrons in its outer shell, needs to share four electrons to achieve a stable octet (eight electrons). This explains its remarkable ability to form four covalent bonds.
Understanding Double Covalent Bonds in Carbon
A double covalent bond is a type of covalent bond where two pairs of electrons are shared between two atoms, typically carbon atoms. This represents a stronger bond than a single covalent bond (where only one electron pair is shared). This enhanced bond strength significantly impacts the properties and reactivity of molecules containing carbon-carbon double bonds.
How it works: Imagine two carbon atoms, each with four electrons in their outer shells. To form a double bond, each carbon atom contributes two electrons to the shared pool, resulting in a total of four shared electrons. These four electrons occupy two bonding molecular orbitals, creating a stronger and shorter bond than a single bond. This double bond is often represented by two parallel lines (=) in chemical structures.
The Geometry of Double Bonds: Planar Structure and Restricted Rotation
Unlike single bonds, which allow free rotation around the bond axis, double bonds exhibit restricted rotation. This is because the two shared electron pairs occupy a planar region between the two carbon atoms, restricting the movement of the atoms relative to each other. The resulting geometry is planar, meaning all atoms involved in the double bond, along with any directly attached atoms, lie in the same plane. This constraint has profound implications on the overall shape and properties of molecules.
Implications of Restricted Rotation: Cis-Trans Isomerism
The restricted rotation around carbon-carbon double bonds gives rise to a phenomenon called cis-trans isomerism (also known as geometric isomerism). Cis-trans isomers are molecules with the same molecular formula and connectivity but differing in the spatial arrangement of their atoms due to the restricted rotation around the double bond.
- Cis isomers: In cis isomers, the similar groups (or substituents) are attached to the same side of the double bond.
- Trans isomers: In trans isomers, the similar groups are attached to opposite sides of the double bond.
Cis-trans isomerism significantly affects the physical and chemical properties of molecules. For example, cis-trans isomers can have different melting points, boiling points, solubilities, and even reactivities. This difference stems from their different shapes and interactions with their surroundings.
Carbon's Role in Diverse Double Bonds
Carbon doesn't just form double bonds with other carbon atoms. It readily forms double bonds with other atoms like oxygen and nitrogen. These double bonds are equally crucial in determining the properties and functions of many organic molecules.
Carbon-Oxygen Double Bonds (C=O)
Carbon-oxygen double bonds (C=O), commonly known as carbonyl groups, are prevalent in many organic functional groups, including aldehydes, ketones, carboxylic acids, and esters. The presence of a carbonyl group significantly affects the molecule's reactivity. The polar nature of the C=O bond (due to the higher electronegativity of oxygen) makes carbonyl compounds susceptible to various nucleophilic attacks, leading to a wide array of chemical reactions.
Carbon-Nitrogen Double Bonds (C=N)
Carbon-nitrogen double bonds (C=N) are found in imines and amides. Imines are characterized by a C=N bond connecting a carbon atom to a nitrogen atom. Amides possess a carbonyl group (C=O) adjacent to a nitrogen atom, contributing to their unique properties and diverse roles in biological systems, including peptide bonds in proteins. The C=N bond's polarity and reactivity patterns differ slightly from C=O bonds, influencing their chemical behaviour.
Double Bonds in Biological Molecules
Double bonds play vital roles in numerous biological molecules, influencing their structure, function, and interactions.
Lipids and Fatty Acids: Unsaturated Fats
Fatty acids, the building blocks of lipids, often contain carbon-carbon double bonds, classifying them as unsaturated fatty acids. The presence and position of these double bonds determine the physical properties of fats and oils. Unsaturated fats, with their double bonds, tend to be liquid at room temperature (oils) due to the kinks introduced into the fatty acid chains by the double bond's geometry. This contrasts with saturated fats, which lack double bonds and are typically solid at room temperature (fats). The different physical states are influenced by the packing efficiency of these molecules, impacted by the geometry conferred by the double bonds.
Vitamins and Hormones: Essential Roles of Double Bonds
Many essential vitamins and hormones contain carbon-carbon double bonds within their structures. These double bonds are crucial for their biological activity. The specific arrangement and number of double bonds dictate the molecular shape, reactivity, and ability to interact with receptors and enzymes, making them effective in their biological roles.
DNA and RNA: The Foundation of Genetics
The nitrogenous bases in DNA and RNA, the molecules responsible for carrying genetic information, contain numerous double bonds, primarily carbon-nitrogen double bonds, within their ring structures. These double bonds contribute to the overall stability and planarity of the bases, enabling them to form precise base pairs through hydrogen bonds and maintain the integrity of the double helix structure in DNA.
Beyond Double Bonds: Exploring Multiple Bonds
While this article focuses primarily on double bonds, it's important to acknowledge the existence of triple bonds in carbon chemistry. A triple bond involves the sharing of three pairs of electrons (six electrons) between two atoms, resulting in an even stronger and shorter bond than a double bond. Triple bonds are typically found in alkynes, compounds featuring carbon-carbon triple bonds. The linear geometry and high reactivity of alkynes make them valuable building blocks in organic synthesis.
Conclusion: The Significance of Double Bonds in Chemistry and Biology
Carbon's capacity to form double covalent bonds significantly influences the diversity and complexity of organic molecules. The restricted rotation around double bonds, the varying reactivity of different double bond types (C=C, C=O, C=N), and their impact on molecular geometry shape the properties and functions of countless organic compounds, from simple hydrocarbons to intricate biological molecules. Understanding the intricacies of carbon's double bonding is fundamental to comprehending the foundations of organic chemistry and the intricate machinery of life itself. The strength, geometry, and reactivity of these bonds continue to fascinate researchers and inspire the development of new materials and technologies. Further research into carbon's bonding prowess promises to unlock even more secrets about the molecular world and its impact on our lives.
Latest Posts
Latest Posts
-
Folder Is To Document As Envelope Is To
Jun 30, 2025
-
How Many Times Does 6 Go Into 100
Jun 30, 2025
-
What Is 453 605 Rounded To The Nearest Thousand
Jun 30, 2025
-
How Many Grams Is A Kilo Of Coke
Jun 30, 2025
-
How Many Tablespoons In A Packet Of Hidden Valley Ranch
Jun 30, 2025
Related Post
Thank you for visiting our website which covers about In A Double Covalent Bond A Carbon Atom Shares . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.