In A Neutralization Reaction And Hydroxide Ions React To Form
Kalali
Mar 17, 2025 · 6 min read
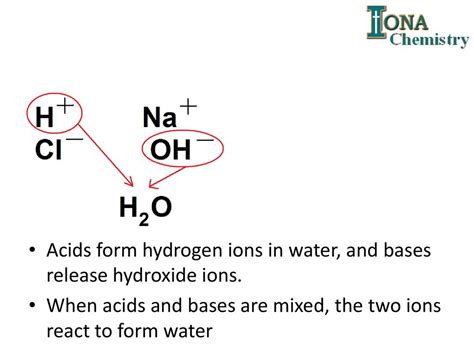
Table of Contents
In a Neutralization Reaction, Hydroxide Ions React to Form Water: A Deep Dive
Neutralization reactions are fundamental chemical processes with far-reaching implications across various fields, from industrial applications to biological systems. At the heart of these reactions lies the interaction between acids and bases, resulting in the formation of a salt and water. This article delves into the specifics of neutralization reactions, focusing on the crucial role of hydroxide ions (OH⁻) and their contribution to water formation. We'll explore the underlying chemistry, different types of neutralization reactions, their applications, and the factors influencing their effectiveness.
Understanding Neutralization Reactions: The Basics
A neutralization reaction is defined as a chemical reaction between an acid and a base, producing a salt and water. The reaction essentially involves the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base to form water (H₂O). The remaining ions from the acid and base combine to form a salt. This process effectively neutralizes the acidic and basic properties of the reactants, resulting in a more neutral solution.
The core equation representing this process is:
Acid + Base → Salt + Water
For instance, the classic reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) demonstrates this perfectly:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Here, the hydrogen ion (H⁺) from HCl and the hydroxide ion (OH⁻) from NaOH combine to form water, while the sodium ion (Na⁺) and chloride ion (Cl⁻) form sodium chloride (NaCl), common table salt.
The Crucial Role of Hydroxide Ions (OH⁻)
Hydroxide ions are the key players in neutralization reactions involving bases. These negatively charged ions are characteristic of Arrhenius bases, which are substances that dissociate in water to release hydroxide ions. The hydroxide ion's strong affinity for hydrogen ions is what drives the neutralization process.
How Hydroxide Ions Form Water
The neutralization reaction fundamentally involves the transfer of a proton (H⁺) from the acid to the hydroxide ion (OH⁻). This proton transfer is the essence of the reaction, leading to the formation of a water molecule. The hydroxide ion accepts the proton, completing its electron shell and forming a stable covalent bond with the hydrogen ion.
The specific reaction of hydroxide ions with hydrogen ions is:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This reaction is highly exothermic, meaning it releases heat. The strength of this reaction is a key factor determining the effectiveness of the neutralization process.
Types of Neutralization Reactions
Neutralization reactions aren't limited to simple acid-base combinations. Several types exist, categorized based on the strength of the acids and bases involved:
1. Strong Acid-Strong Base Neutralization:
This involves the reaction between a strong acid (like HCl, H₂SO₄, HNO₃) and a strong base (like NaOH, KOH, Ca(OH)₂). These reactions proceed to completion, meaning almost all the acid and base react to form water and salt. The resulting solution is usually neutral (pH 7) at the equivalence point.
2. Weak Acid-Strong Base Neutralization:
Here, a weak acid (like acetic acid, CH₃COOH) reacts with a strong base. Weak acids don't fully dissociate in water, resulting in an equilibrium between the undissociated acid and its ions. The neutralization reaction is less complete, and the resulting solution at the equivalence point will be slightly basic (pH > 7) due to the presence of the conjugate base of the weak acid.
3. Strong Acid-Weak Base Neutralization:
This involves a strong acid reacting with a weak base (like ammonia, NH₃). Similar to the previous case, the reaction is not complete, and the resulting solution at the equivalence point will be slightly acidic (pH < 7) due to the presence of the conjugate acid of the weak base.
4. Weak Acid-Weak Base Neutralization:
This is the least straightforward type of neutralization. Both the acid and base are weak, leading to a complex equilibrium system. Predicting the pH at the equivalence point requires considering the relative strengths of the acid and base and their respective Ka and Kb values.
Applications of Neutralization Reactions
Neutralization reactions are ubiquitous, finding application in diverse fields:
1. Industrial Processes:
- Wastewater Treatment: Neutralization is crucial for treating industrial wastewater, adjusting its pH to environmentally acceptable levels before discharge. Acids or bases are added to neutralize excessive alkalinity or acidity, respectively.
- Chemical Synthesis: Many chemical syntheses involve neutralization steps to control the reaction environment and obtain the desired product.
- Food and Beverage Industry: pH control is vital in food processing and preservation. Neutralization reactions are used to adjust the acidity or alkalinity of food products.
2. Biological Systems:
- Maintaining Blood pH: The human body meticulously maintains the pH of blood within a narrow range. Buffer systems, which involve neutralization reactions, play a critical role in preventing drastic pH fluctuations.
- Digestion: The stomach uses hydrochloric acid to digest food. Neutralization reactions in the small intestine, utilizing bicarbonate ions, help regulate the pH, preventing damage to the intestinal lining.
3. Everyday Life:
- Antacids: Antacids contain bases that neutralize excess stomach acid, relieving heartburn and indigestion.
- Cleaning Products: Many cleaning products utilize acids or bases to remove stains and grease. Neutralization reactions help restore the cleaned surfaces to a neutral pH.
Factors Affecting Neutralization Reactions
Several factors influence the effectiveness and outcome of neutralization reactions:
1. Concentration of Reactants:
Higher concentrations of acid and base lead to faster and more complete neutralization.
2. Temperature:
Increased temperature generally accelerates the reaction rate.
3. Presence of Catalysts:
Certain catalysts can enhance the reaction rate without being consumed in the process.
4. Nature of the Acid and Base:
The strength of the acid and base significantly impacts the completeness of the reaction and the pH of the resulting solution.
Titration: Quantifying Neutralization
Titration is a laboratory technique used to determine the concentration of an unknown acid or base by reacting it with a solution of known concentration. Using an indicator, such as phenolphthalein, the equivalence point – when the acid and base have completely neutralized each other – is identified, allowing the calculation of the unknown concentration.
Conclusion: The Importance of Hydroxide Ions in Neutralization
In conclusion, hydroxide ions are indispensable components of neutralization reactions involving bases. Their ability to accept protons from acids drives the formation of water, the hallmark of this fundamental chemical process. Understanding the role of hydroxide ions and the various types of neutralization reactions is crucial across numerous scientific and industrial applications, from regulating biological processes to managing environmental impacts. The detailed study of neutralization reactions, particularly concerning the behavior of hydroxide ions, provides invaluable insights into chemical equilibrium, reaction kinetics, and a broad range of practical applications. Further research continues to refine our understanding of these crucial reactions and their applications in various contexts.
Latest Posts
Latest Posts
-
What Is 1 5 8 As A Decimal
Mar 17, 2025
-
3 Feet 6 Inches In Cm
Mar 17, 2025
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about In A Neutralization Reaction And Hydroxide Ions React To Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
