Is Acetylene A Pure Substance Or Mixture
Kalali
Mar 17, 2025 · 5 min read
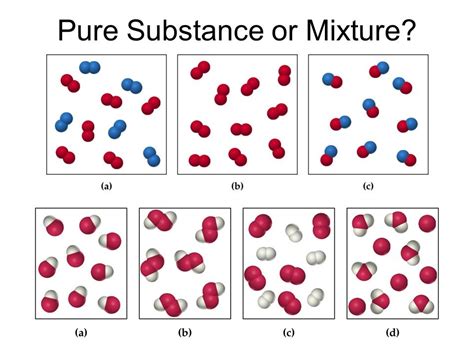
Table of Contents
Is Acetylene a Pure Substance or a Mixture? A Comprehensive Look
Acetylene, a simple yet fascinating hydrocarbon, often sparks the question: is it a pure substance or a mixture? The answer, while seemingly straightforward, requires a deeper understanding of its chemical properties, production methods, and potential contaminants. This comprehensive article will delve into the intricacies of acetylene's composition, addressing this question definitively and exploring related concepts.
Understanding Pure Substances and Mixtures
Before classifying acetylene, let's establish a clear understanding of the terms "pure substance" and "mixture."
Pure Substance: A pure substance is a form of matter that has a constant composition and properties throughout its mass. It cannot be separated into other substances through physical methods. Pure substances can be elements (like oxygen or iron) or compounds (like water or salt). They have a defined melting point and boiling point.
Mixture: A mixture is a combination of two or more pure substances that are not chemically bonded. Mixtures can be homogeneous (uniform composition, like saltwater) or heterogeneous (non-uniform composition, like sand and water). Mixtures can be separated into their components using physical methods like filtration, distillation, or chromatography. Mixtures typically have a range of melting and boiling points.
The Chemical Nature of Acetylene
Acetylene, chemically known as ethyne, is a hydrocarbon with the chemical formula C₂H₂. It's the simplest alkyne, characterized by a triple bond between the two carbon atoms. This triple bond significantly influences its reactivity and properties. In its purest form, acetylene consists exclusively of these carbon and hydrogen atoms in a 1:1 ratio. This fixed ratio is a key characteristic of a compound, not a mixture.
Ideal vs. Real-World Acetylene
Theoretically, pure acetylene should consist solely of C₂H₂ molecules. However, in practice, commercially available acetylene is rarely 100% pure. The production process and storage methods introduce potential impurities. This is where the complexity arises in answering the initial question.
Acetylene Production and Potential Impurities
Acetylene is primarily produced through two methods:
1. Calcium Carbide Method: This traditional method involves reacting calcium carbide (CaC₂) with water. The reaction produces acetylene gas and calcium hydroxide (Ca(OH)₂). This process can introduce impurities such as:
- Phosphine (PH₃): A highly toxic and flammable gas.
- Hydrogen Sulfide (H₂S): Another toxic and flammable gas with a characteristic rotten egg smell.
- Ammonia (NH₃): A pungent-smelling gas.
- Moisture (H₂O): Water vapor can react with acetylene under certain conditions.
- Residual Calcium Carbide: Unreacted calcium carbide particles.
The presence of these impurities significantly impacts the purity of the acetylene gas produced.
2. Partial Oxidation of Hydrocarbons: Modern methods often employ partial oxidation of hydrocarbons like methane or naphtha. While this method can yield higher purity acetylene, trace amounts of other hydrocarbons and byproducts can still remain. These might include:
- Other Alkenes and Alkynes: Unsaturated hydrocarbons with different chain lengths.
- Unreacted Hydrocarbons: Starting materials that didn't fully react.
- Carbon Monoxide (CO): A toxic gas.
- Carbon Dioxide (CO₂): A non-toxic gas but still an impurity.
Purification of Acetylene
To ensure safe and efficient use, acetylene undergoes purification processes. These processes aim to remove the impurities mentioned above. Common techniques include:
- Scrubbing: Removing impurities by passing the gas through a series of scrubbing towers containing specific solvents to absorb unwanted gases.
- Compression and Cooling: Condensing and separating impurities based on their boiling points.
- Filtration: Removing solid particles like unreacted calcium carbide.
Even after purification, trace amounts of impurities might persist. The level of purity depends on the production method, the purification techniques employed, and the intended application.
The "Pure" Acetylene Paradox
This leads us back to the initial question: is acetylene a pure substance or a mixture? The answer is nuanced.
In its ideal, theoretical state, acetylene is a pure substance – a compound composed solely of C₂H₂ molecules. Its fixed chemical formula and defined properties support this classification.
However, commercially available acetylene is invariably a mixture. While purification processes strive for high purity, trace amounts of impurities inevitably remain. The extent of these impurities determines the level of "purity" of the acetylene and consequently shifts its classification from a pure substance to a mixture.
Implications of Impurities
The presence of impurities in acetylene has significant implications:
- Safety: Impurities like phosphine, hydrogen sulfide, and carbon monoxide are highly toxic and flammable, increasing the risk of accidents during handling and use.
- Performance: Impurities can affect the efficiency of acetylene in applications like welding and cutting. They can alter the flame characteristics and potentially lead to substandard welds.
- Storage: Some impurities can react with acetylene, leading to instability and potential hazards during storage.
Conclusion: A Matter of Perspective
The question of whether acetylene is a pure substance or a mixture hinges on the perspective. From a purely chemical standpoint, acetylene (C₂H₂) is a pure substance – a compound. However, in practical terms, commercially available acetylene is always a mixture due to the inevitable presence of impurities from its production and handling. The level of purity, and therefore the degree to which it deviates from a pure substance, is dependent on the specific manufacturing and purification process employed. Understanding this distinction is crucial for safe and effective handling of this important industrial gas. Further research into more efficient purification techniques is crucial to minimize these impurities and bring the commercial product closer to the ideal pure substance. The ongoing quest for higher purity acetylene underscores the continuous evolution in industrial chemistry and safety standards.
Latest Posts
Latest Posts
-
Cuanto Es 36 Grados Fahrenheit En Centigrados
Mar 18, 2025
-
How Many Cups In 48 Oz
Mar 18, 2025
-
How Long Is 60 Cm In Inches
Mar 18, 2025
-
How Many Ounces In 200 Ml
Mar 18, 2025
-
How Many Ounces Is 10 Cups
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Acetylene A Pure Substance Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
