Is An Atom Smaller Than A Cell
Kalali
Mar 15, 2025 · 6 min read
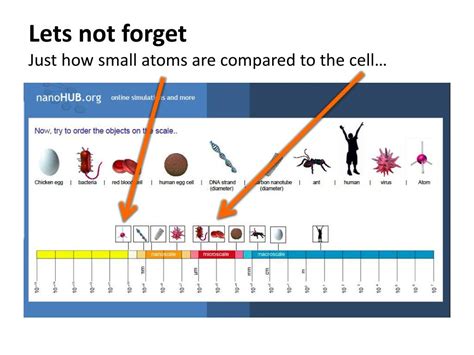
Table of Contents
Is an Atom Smaller Than a Cell? A Deep Dive into the Microscopic World
The question, "Is an atom smaller than a cell?" might seem trivial at first glance. The answer, a resounding yes, forms the foundation of our understanding of the biological and physical worlds. However, exploring the vast difference in scale between these two fundamental units of matter reveals a captivating journey into the intricacies of life and the universe. This article will delve into the specifics of atoms and cells, highlighting their individual structures, functions, and the significant size disparity between them. We'll also explore the fascinating world bridging the gap between these two incredibly different structures.
Understanding Atoms: The Building Blocks of Matter
Atoms are the fundamental building blocks of all matter. Everything around us, from the air we breathe to the Earth beneath our feet, is composed of atoms. These tiny particles are incredibly small, far beyond the limits of visibility with even the most powerful optical microscopes. Their size is measured in angstroms (Å), where 1 Å is equal to 0.1 nanometers (nm). A single atom's diameter typically ranges from 0.1 to 0.5 nm.
Atomic Structure: A Subatomic Perspective
An atom consists of a central nucleus containing positively charged protons and electrically neutral neutrons. Surrounding the nucleus is a cloud of negatively charged electrons that orbit the nucleus at various energy levels or shells. The number of protons in the nucleus determines the atomic number of an element and defines its chemical properties. Isotopes of an element differ in the number of neutrons.
Different atoms combine to form molecules, the next level of organization in matter. The way atoms bond together – either through covalent, ionic, or metallic bonds – dictates the properties of the resulting molecules. Water (H₂O), for example, is a molecule formed from two hydrogen atoms and one oxygen atom. The unique properties of water are a direct consequence of the atomic structure and bonding of its constituent atoms. This intricate interplay of subatomic particles defines the building blocks of all substances.
Understanding Cells: The Fundamental Units of Life
Cells are the basic structural and functional units of all living organisms. From the simplest single-celled bacteria to complex multicellular organisms like humans, life is organized around the cell. Compared to atoms, cells are macroscopic giants, measurable in micrometers (µm). A typical human cell might measure 10-100 µm in diameter, highlighting the massive size difference compared to atoms.
Cell Structure and Function: A Complex Organization
Cells exhibit remarkable complexity in their structure and function. They are typically enclosed by a plasma membrane, a selectively permeable barrier that regulates the passage of substances into and out of the cell. Inside the cell, a variety of organelles perform specialized functions. These include:
- Nucleus: Contains the cell's genetic material (DNA).
- Mitochondria: The "powerhouses" of the cell, generating energy (ATP).
- Ribosomes: Sites of protein synthesis.
- Endoplasmic Reticulum: A network of membranes involved in protein and lipid synthesis.
- Golgi Apparatus: Modifies and packages proteins for transport.
- Lysosomes: Digest cellular waste.
The specific types and arrangement of organelles vary depending on the cell type and the organism. Prokaryotic cells (bacteria and archaea) lack a nucleus and other membrane-bound organelles, while eukaryotic cells (plants, animals, fungi, and protists) possess a well-defined nucleus and a complex array of organelles. The intricate workings within the cell dictate the overall functionality of the organism.
The Vast Difference in Scale: Atoms vs. Cells
The difference in size between atoms and cells is staggering. To appreciate the scale, consider the following:
- Atoms: Measured in angstroms (Å) or nanometers (nm), typically ranging from 0.1 to 0.5 nm in diameter.
- Cells: Measured in micrometers (µm), typically ranging from 10 to 100 µm in diameter.
This means that a typical cell is millions of times larger than a typical atom. Imagine a single grain of sand – that’s still much, much larger than a single cell, and a cell is still vastly larger than an atom. This vast difference in scale is essential to understand the hierarchy of biological organization.
Bridging the Gap: From Atoms to Molecules to Cells
The journey from the incredibly small world of atoms to the relatively large world of cells involves a fascinating progression of levels of organization. Atoms combine to form molecules, which in turn assemble into larger structures, eventually forming the complex machinery of a cell. Here's a step-by-step breakdown:
- Atoms: The fundamental building blocks of matter.
- Molecules: Formed by the bonding of atoms. Examples include water (H₂O), glucose (C₆H₁₂O₆), and proteins (complex chains of amino acids).
- Macromolecules: Large, complex molecules like proteins, carbohydrates, lipids, and nucleic acids. These form the structural components and functional machinery of cells.
- Organelles: Membrane-bound compartments within cells performing specific functions (e.g., mitochondria, nucleus, ribosomes).
- Cells: The basic units of life, containing all the necessary components to carry out life processes.
This hierarchical organization demonstrates how the simple building blocks of matter – atoms – contribute to the remarkable complexity and diversity of life. The intricate interplay between atoms, molecules, and macromolecules ultimately gives rise to the structure and function of cells.
The Significance of the Size Difference
The substantial size difference between atoms and cells has profound implications for our understanding of the biological world. The vast space within a cell allows for:
- Compartmentalization: Organelles are separated from each other, allowing for specialized functions to occur without interference.
- Efficient Transport: Molecules can move efficiently throughout the cell via various transport mechanisms.
- Complex Chemical Reactions: The cellular environment provides the necessary space and conditions for a wide range of biochemical reactions to take place.
Without this vast scale difference, the intricate processes of life, as we know them, would be impossible.
Conclusion: A Microscopic Marvel
The simple answer to the question, "Is an atom smaller than a cell?" is a definitive yes. However, the exploration of the vast difference in scale between these two units reveals a remarkable journey into the fundamental building blocks of matter and the intricate complexity of life. From the subatomic particles within atoms to the intricate machinery within cells, the story unfolds as a testament to the elegance and sophistication of the natural world. Understanding this size discrepancy is crucial for grasping the hierarchical organization of life and appreciating the incredible power of nature at both the microscopic and macroscopic levels. The vastness of the difference underlines the complexity of biology and the fascinating interplay of different levels of organization in living organisms. The journey from atom to cell is a testament to the intricate design of life and the incredible power of nature’s building blocks.
Latest Posts
Latest Posts
-
Is Sound Kinetic Or Potential Energy
Mar 15, 2025
-
How Many Feet In 150 Inches
Mar 15, 2025
-
Is Grass A Producer Or Consumer
Mar 15, 2025
-
Organisms That Cannot Make Their Own Food Are Called
Mar 15, 2025
-
Cuanto Es El 3 Por Ciento De 1000
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is An Atom Smaller Than A Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
