Is Ba Oh 2 A Strong Base
Kalali
Mar 14, 2025 · 4 min read
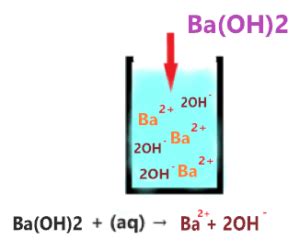
Table of Contents
Is Ba(OH)₂ a Strong Base? A Comprehensive Look
Barium hydroxide, Ba(OH)₂, is a common inorganic compound frequently encountered in chemistry. Understanding its properties, particularly its basicity, is crucial for various applications, from industrial processes to laboratory experiments. This article will delve into the question: Is Ba(OH)₂ a strong base? We'll explore its definition, properties, applications, and compare it to other bases to offer a comprehensive answer.
Understanding Strong Bases
Before classifying barium hydroxide, let's define what constitutes a strong base. A strong base is a substance that completely dissociates in water, releasing hydroxide ions (OH⁻) into the solution. This complete dissociation leads to a high concentration of OH⁻ ions, resulting in a significantly high pH value (typically above 7, and often much higher). The higher the concentration of OH⁻ ions, the stronger the base.
The Dissociation of Barium Hydroxide
Barium hydroxide, when dissolved in water, undergoes complete dissociation:
Ba(OH)₂(s) → Ba²⁺(aq) + 2OH⁻(aq)
This equation clearly shows that one mole of Ba(OH)₂ produces two moles of hydroxide ions (OH⁻). This high yield of hydroxide ions is a key characteristic of strong bases. The complete dissociation ensures a significant increase in the hydroxide ion concentration in the solution, leading to a highly alkaline environment.
Factors Influencing Basicity
While Ba(OH)₂ generally behaves as a strong base, certain factors can subtly influence its basicity:
1. Concentration:
The concentration of Ba(OH)₂ in the solution directly impacts its basicity. A higher concentration leads to a higher concentration of OH⁻ ions, resulting in a stronger alkaline solution. A dilute solution will still be basic, but its pH will be lower than a concentrated solution.
2. Temperature:
Temperature can influence the solubility of Ba(OH)₂, which in turn affects the concentration of OH⁻ ions. Generally, solubility increases with temperature, meaning a higher temperature can lead to a slightly stronger basic solution, although the effect is not as dramatic as with concentration changes.
3. Solvation Effects:
The solvent used can affect the dissociation and, consequently, the strength of the base. While water is the most common solvent, other solvents can impact the degree of dissociation, possibly slightly altering the apparent basicity of Ba(OH)₂.
Comparing Ba(OH)₂ to Other Bases
To further solidify the understanding of Ba(OH)₂'s strength, it's helpful to compare it with other bases:
Strong Bases:
- NaOH (Sodium Hydroxide): NaOH is a classic example of a strong base, completely dissociating in water to produce Na⁺ and OH⁻ ions. It is comparable to Ba(OH)₂ in its strength.
- KOH (Potassium Hydroxide): Similar to NaOH, KOH is another strong base with complete dissociation in water. Its strength is also comparable to Ba(OH)₂.
- LiOH (Lithium Hydroxide): Although slightly less soluble than NaOH and KOH, LiOH is still considered a strong base due to its complete dissociation.
- Ca(OH)₂ (Calcium Hydroxide): While considered a strong base due to complete dissociation, Ca(OH)₂ has lower solubility than Ba(OH)₂, leading to a lower concentration of OH⁻ ions in saturated solutions. Therefore, its effective basicity might be slightly lower than Ba(OH)₂ at similar concentrations.
Weak Bases:
- NH₃ (Ammonia): Ammonia is a weak base, meaning it only partially dissociates in water. It doesn't produce a high concentration of OH⁻ ions compared to strong bases.
- CH₃COOH (Acetic Acid): Acetic acid is a weak acid, its conjugate base (acetate ion) is a weak base. It also only partially dissociates.
The key difference between strong bases like Ba(OH)₂ and weak bases like ammonia lies in the extent of dissociation. Strong bases completely dissociate, while weak bases only partially dissociate.
Applications of Barium Hydroxide
The strong basicity of Ba(OH)₂ makes it useful in several applications:
- Industrial Processes: Ba(OH)₂ is used in various industrial processes, including sugar refining, where it helps to remove impurities. Its strong alkalinity is crucial for these applications.
- Water Treatment: Ba(OH)₂ can be used in water treatment to adjust pH and remove contaminants. Its ability to neutralize acids and precipitate certain ions makes it useful in water purification.
- Chemical Synthesis: As a strong base, it serves as a reactant or catalyst in various chemical reactions.
- Laboratory Reagent: In laboratories, it's used in titrations and other analytical procedures where a strong base is required.
Safety Considerations
It's crucial to handle Ba(OH)₂ with care due to its corrosive nature. Direct contact with skin and eyes should be avoided. Appropriate safety measures, such as wearing gloves and eye protection, are essential when working with barium hydroxide. Proper disposal methods should also be followed.
Conclusion: Ba(OH)₂ is a Strong Base
In summary, yes, Ba(OH)₂ is considered a strong base. Its complete dissociation in water produces a high concentration of hydroxide ions, leading to a highly alkaline solution. While factors like concentration and temperature can influence its apparent strength, its inherent ability to fully dissociate firmly places it within the category of strong bases. Its comparison to other strong and weak bases further reinforces this classification. Understanding this property is essential for its safe and effective use in various applications, from industrial processes to laboratory experiments. Always remember to handle this compound with appropriate safety precautions.
Latest Posts
Latest Posts
-
1 Pound Pasta Is How Many Cups
Jul 04, 2025
-
How Many Cans Of Soda Are In A 2 Liter
Jul 04, 2025
-
How Do U Say Of In Spanish
Jul 04, 2025
-
How Many Oz In A 10 Can
Jul 04, 2025
-
How Many Cups Is 16 Ounces Of Peanut Butter
Jul 04, 2025
Related Post
Thank you for visiting our website which covers about Is Ba Oh 2 A Strong Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.