Is Baking Soda An Element Or Compound
Kalali
Mar 12, 2025 · 5 min read
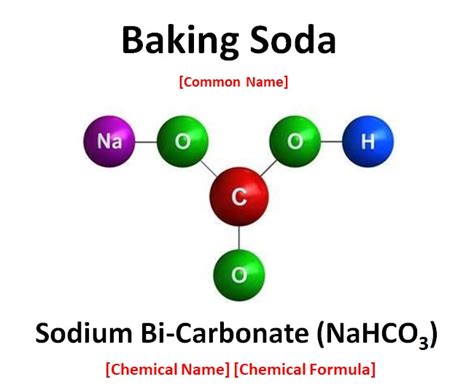
Table of Contents
Is Baking Soda an Element or Compound? A Deep Dive into Chemical Composition
Baking soda, a ubiquitous ingredient in countless kitchens worldwide, is more than just a leavening agent. Understanding its chemical nature – whether it's an element or a compound – opens the door to appreciating its diverse applications and fascinating chemical properties. This comprehensive article delves deep into the composition of baking soda, exploring its elemental makeup, chemical structure, and the key distinctions between elements and compounds. We'll also examine its unique properties and how its chemical structure dictates its functionality.
Understanding the Basics: Elements vs. Compounds
Before we dive into the specifics of baking soda, let's clarify the fundamental difference between elements and compounds.
Elements: The Building Blocks of Matter
Elements are pure substances that cannot be broken down into simpler substances by chemical means. They are the fundamental building blocks of all matter and are represented by unique symbols on the periodic table. Examples include oxygen (O), hydrogen (H), and carbon (C). Each element is defined by its unique number of protons in its atomic nucleus, which determines its atomic number and its place on the periodic table.
Compounds: A Union of Elements
Compounds are substances formed when two or more different elements combine chemically in fixed proportions. These elements are bound together by chemical bonds, creating a new substance with distinct properties from its constituent elements. Water (H₂O), for example, is a compound composed of two hydrogen atoms and one oxygen atom. The properties of water are vastly different from those of hydrogen and oxygen in their elemental forms.
Baking Soda: Unveiling the Chemical Identity
Now, let's focus on baking soda, also known as sodium bicarbonate. Its chemical formula is NaHCO₃. This formula immediately reveals its identity as a compound, not an element.
Deconstructing the Formula: Sodium Bicarbonate (NaHCO₃)
The formula NaHCO₃ tells us that baking soda is composed of:
- Na (Sodium): An alkali metal, highly reactive in its pure form.
- H (Hydrogen): A non-metal, the lightest element in the universe.
- C (Carbon): A non-metal, crucial for organic life.
- O₃ (Three Oxygen Atoms): A non-metal, vital for respiration and numerous chemical processes.
These four different elements combine in a specific ratio to form sodium bicarbonate. This precise ratio is what distinguishes baking soda from other compounds. The chemical bonds between these atoms are ionic and covalent, providing stability to the molecule.
The Ionic and Covalent Bonds in Baking Soda
The structure of sodium bicarbonate involves both ionic and covalent bonds. The sodium (Na) atom forms an ionic bond with the bicarbonate (HCO₃) ion. This ionic bond is formed due to the transfer of an electron from the sodium atom to the bicarbonate ion. Within the bicarbonate ion itself, covalent bonds exist between the carbon, hydrogen, and oxygen atoms. These covalent bonds involve the sharing of electrons between these atoms.
Why Baking Soda Isn't an Element
The presence of multiple different elements chemically bonded together definitively classifies baking soda as a compound, not an element. Elements are indivisible by chemical means; you cannot break down sodium bicarbonate into sodium, hydrogen, carbon, and oxygen through simple chemical reactions without significant energy input. Specialized processes like electrolysis are needed to separate the components of baking soda. This inability to be broken down into simpler substances by ordinary chemical means is the defining characteristic that separates elements from compounds.
Properties of Baking Soda: A Consequence of its Chemical Structure
The unique properties of baking soda are directly linked to its chemical structure and composition.
Leavening Agent: The Magic of Carbon Dioxide
Baking soda's most famous application is as a leavening agent in baking. When baking soda reacts with an acid (like vinegar or lemon juice), it produces carbon dioxide gas (CO₂). This gas expands, creating air pockets in the batter, leading to the rise and lightness of baked goods. The chemical reaction is represented as follows:
NaHCO₃ + H⁺ → Na⁺ + H₂O + CO₂
This reaction highlights how the chemical composition of baking soda allows it to participate in this crucial reaction in baking.
Mild Abrasive: Gentle Cleaning Power
The mildly abrasive nature of baking soda makes it a popular cleaning agent. Its tiny particles can gently scrub surfaces without causing significant damage. This is a physical property linked to its crystalline structure.
Neutralizing Agent: Balancing pH Levels
Baking soda's ability to neutralize acids makes it useful in various applications, including controlling acidity in the stomach (antacids) and regulating pH levels in pools and gardens. This neutralization reaction is a direct result of its chemical ability to react with acids, forming water and salt.
Deodorizer: Absorbing Unpleasant Odors
Baking soda's ability to absorb odors is due to its large surface area and its capacity to trap odor-causing molecules within its crystal structure. This is a physical phenomenon, but its effectiveness is strongly influenced by the chemical properties of baking soda.
Baking Soda vs. Other Compounds: Emphasizing the Distinction
It is crucial to differentiate baking soda from other commonly confused substances. For example, baking powder contains baking soda, but also an acid and a drying agent. Baking powder is a more complex mixture than baking soda alone. Washing soda (sodium carbonate, Na₂CO₃) is a different compound altogether with different properties and uses. Understanding these distinctions is vital for correct application in various contexts.
Conclusion: Baking Soda – A Compound with Diverse Applications
In conclusion, baking soda is unequivocally a compound, not an element. Its chemical formula, NaHCO₃, clearly indicates that it is composed of four different elements – sodium, hydrogen, carbon, and oxygen – chemically bonded together. Its unique properties, from its leavening capabilities in baking to its cleaning and deodorizing abilities, all stem from this chemical composition. Understanding the chemical nature of baking soda allows for a deeper appreciation of its versatile applications and highlights the fundamental principles of chemistry at work in everyday life. This knowledge can enhance our understanding of chemical reactions and processes, ultimately leading to a more informed and efficient use of this remarkable compound. The intricate interplay of ionic and covalent bonding, along with its reactivity with acids, dictates the diverse functionality of baking soda in our everyday lives, from the kitchen to cleaning and beyond. The seemingly simple compound is a testament to the power of chemical structure and its impact on functionality.
Latest Posts
Latest Posts
-
Can You Be A Professor With A Masters
Jul 14, 2025
-
How Many Bottles Of Water Is 1 Liter
Jul 14, 2025
-
How Many Days In A Million Minutes
Jul 14, 2025
-
How Many Days Is In 11 Weeks
Jul 14, 2025
-
How Many Grams Are In One Tola Gold
Jul 14, 2025
Related Post
Thank you for visiting our website which covers about Is Baking Soda An Element Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.