Is Boiling Point Intensive Or Extensive
Kalali
Mar 26, 2025 · 5 min read
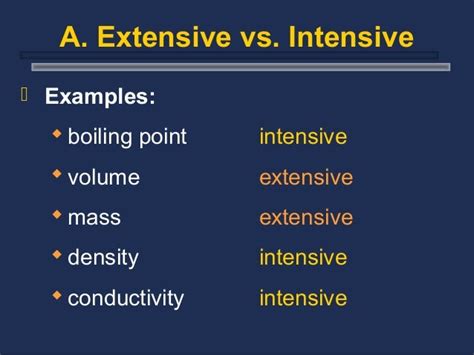
Table of Contents
Is Boiling Point Intensive or Extensive? Understanding Physical Properties of Matter
The question of whether boiling point is an intensive or extensive property is a fundamental concept in chemistry and physics. Understanding this distinction is crucial for interpreting experimental data and predicting the behavior of substances. This comprehensive guide delves deep into the nature of boiling point, exploring its definition, the difference between intensive and extensive properties, and definitively answering the central question. We'll also touch upon related concepts and practical applications.
Intensive vs. Extensive Properties: A Clear Distinction
Before we classify boiling point, let's clearly define the difference between intensive and extensive properties:
Intensive Properties: These properties are independent of the amount of substance present. They remain constant regardless of whether you have a small sample or a large quantity. Examples include:
- Temperature: The temperature of a cup of water is the same as the temperature of a swimming pool full of water, assuming they are both at the same temperature.
- Density: The density of gold remains the same whether you have a gold nugget or a gold bar.
- Boiling Point: This is the focus of our article, and we will explore this in detail.
- Melting Point: Similar to boiling point, it's unaffected by the amount of substance.
- Color: The color of a substance remains constant regardless of the amount.
Extensive Properties: These properties do depend on the amount of substance. They change proportionally with the size of the sample. Examples include:
- Mass: A kilogram of water has a greater mass than a gram of water.
- Volume: A liter of water occupies a larger volume than a milliliter of water.
- Heat Capacity: The amount of heat required to raise the temperature of a substance depends on its mass.
- Length: A longer piece of metal has a greater length than a shorter piece.
- Total Energy: The total energy contained within a system increases with the amount of matter.
Boiling Point: A Deep Dive
The boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure surrounding the liquid. At this point, bubbles of vapor form within the liquid and rise to the surface, resulting in the liquid transitioning to the gaseous phase.
It's crucial to understand that the boiling point is dependent on pressure. At higher altitudes, where atmospheric pressure is lower, the boiling point of water is lower (around 90°C or 194°F at the top of Mount Everest, as opposed to 100°C or 212°F at sea level). Conversely, at higher pressures, the boiling point increases. Pressure cookers utilize this principle to cook food faster at higher temperatures.
The Crucial Role of Intermolecular Forces
The boiling point of a substance is significantly influenced by the strength of its intermolecular forces. Stronger intermolecular forces (like hydrogen bonding, dipole-dipole interactions, or London dispersion forces) require more energy to overcome, resulting in a higher boiling point. For instance, water, with its strong hydrogen bonds, has a relatively high boiling point compared to substances with weaker intermolecular forces.
Is Boiling Point Intensive or Extensive? The Definitive Answer
Boiling Point is an Intensive Property.
The boiling point of a pure substance remains constant regardless of the amount of substance present. Whether you have a single drop or a liter of pure water, under the same pressure conditions, it will boil at the same temperature. This is the defining characteristic of an intensive property.
The amount of water present does not alter the temperature at which boiling occurs. This independence from quantity makes boiling point an intensive property. It's solely determined by the intrinsic nature of the substance and the external pressure.
Practical Applications and Implications
The intensive nature of the boiling point has wide-ranging practical implications in various fields:
- Chemistry: Boiling point is a crucial physical property used to identify and characterize unknown substances. It helps in purification through distillation techniques, separating components based on their differing boiling points.
- Food Science: Understanding the boiling point of water at different altitudes is essential for adjusting cooking times and techniques.
- Engineering: Boiling points are vital in designing systems that involve phase transitions, such as refrigeration and power generation.
- Meteorology: The boiling point of water plays a role in weather patterns and atmospheric processes.
Common Misconceptions
One common misconception arises from confusing the boiling point with the amount of energy required to completely boil a sample. The total heat energy needed is extensive; it depends on the mass of the substance. However, the temperature at which boiling begins (the boiling point itself) remains an intensive property. You need more energy to boil a larger quantity, but the temperature remains the same.
Related Concepts and Further Exploration
- Normal Boiling Point: This refers to the boiling point of a substance at a standard pressure of 1 atmosphere (101.325 kPa).
- Phase Diagrams: These diagrams illustrate the relationship between pressure, temperature, and the phases of a substance, providing a visual representation of boiling points under varying conditions.
- Clausius-Clapeyron Equation: This equation describes the relationship between the vapor pressure of a liquid and its temperature, allowing for the calculation of boiling points at different pressures.
- Critical Point: This is the temperature and pressure above which a substance cannot exist as a liquid, regardless of the pressure.
Conclusion: A Clear Understanding of Boiling Point
In conclusion, the boiling point is definitively an intensive property. It is a fundamental physical characteristic of a substance, independent of the amount of matter present. Understanding this distinction is crucial for various scientific disciplines and applications, allowing for accurate predictions and effective utilization of this important physical property. The boiling point, while influenced by external pressure and intermolecular forces, remains a constant for a given pure substance under specific pressure conditions, solidifying its classification as an intensive property. This inherent characteristic makes it a valuable tool in identifying, purifying, and understanding the behavior of different materials.
Latest Posts
Latest Posts
-
The Cost Of A Single Ticket Depends On
Mar 29, 2025
-
What Is 55 F In Celsius
Mar 29, 2025
-
What Is 18 25 As A Percentage
Mar 29, 2025
-
29 C Is What In Fahrenheit
Mar 29, 2025
-
Cuanto Es 52 Fahrenheit En Centigrados
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Point Intensive Or Extensive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
