Is Boiling Water Endothermic Or Exothermic
Kalali
Mar 10, 2025 · 5 min read
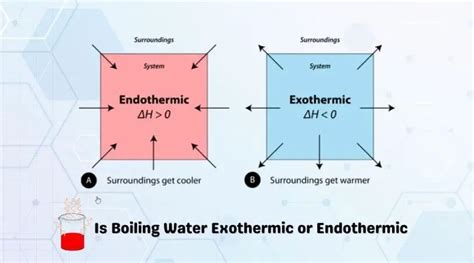
Table of Contents
Is Boiling Water Endothermic or Exothermic? Understanding Heat Transfer in Phase Changes
The question of whether boiling water is an endothermic or exothermic process might seem straightforward, but a deeper understanding requires exploring the nuances of heat transfer and phase changes. While the simple answer might seem obvious, the underlying principles are crucial for grasping fundamental concepts in chemistry and physics. This comprehensive article will delve into the intricacies of this process, examining the definitions of endothermic and exothermic reactions, the role of heat energy in boiling water, and related concepts.
Understanding Endothermic and Exothermic Reactions
Before tackling the specifics of boiling water, let's clarify the fundamental definitions:
Endothermic reactions absorb heat energy from their surroundings. Think of it like a sponge soaking up water; the system's energy increases. The temperature of the surroundings will decrease as heat is transferred into the system. Examples include melting ice and photosynthesis.
Exothermic reactions, on the other hand, release heat energy into their surroundings. This is akin to a fire; the system's energy decreases, and the temperature of the surroundings increases. Examples include combustion and the neutralization of acids and bases.
The Boiling Point: A Critical Transition
Water boils at 100 degrees Celsius (212 degrees Fahrenheit) at standard atmospheric pressure. This boiling point represents the temperature at which the liquid water transitions into gaseous water vapor (steam). This phase transition is the key to understanding the endothermic or exothermic nature of the process.
The Role of Heat Energy in Boiling
To boil water, you need to supply heat energy. This heat isn't simply raising the temperature of the water; it's breaking the intermolecular bonds that hold the water molecules together in the liquid state. These bonds, primarily hydrogen bonds, require a significant amount of energy to overcome.
As you heat water, the kinetic energy of the water molecules increases, causing them to move faster. Once the boiling point is reached, the added heat energy is used to overcome the attractive forces between molecules, allowing them to escape from the liquid phase and transition into the gaseous phase. This is why the temperature of boiling water remains constant despite continuous heating—all the added energy is going into the phase change, not into raising the temperature further.
Boiling Water: An Endothermic Process
Therefore, boiling water is unequivocally an endothermic process. The system (the water) absorbs heat energy from its surroundings (the heat source, like a stove or Bunsen burner). The surroundings lose energy as this heat is absorbed by the water to drive the phase transition from liquid to gas. This absorption of energy is characteristic of an endothermic reaction.
Visualizing the Energy Change
Imagine a pot of water on a stove. The heat from the stove transfers energy to the water. This energy increases the kinetic energy of the water molecules. As the water heats, it absorbs more and more energy. Once boiling begins, the temperature remains at 100°C (at standard atmospheric pressure), but the water continues to absorb heat energy, utilizing it to break the intermolecular hydrogen bonds and convert liquid water to steam.
Beyond Boiling: Latent Heat of Vaporization
The amount of heat required to change one gram of a substance from liquid to gas at its boiling point is called the latent heat of vaporization. For water, this value is relatively high (approximately 2260 J/g), indicating that a substantial amount of energy is required to overcome the intermolecular forces and convert liquid water into steam. This high latent heat of vaporization contributes to water's important role in regulating temperature on Earth.
Factors Affecting Boiling Point
Several factors influence the boiling point of water, and understanding these factors provides a more complete picture of the endothermic process:
-
Atmospheric Pressure: Higher atmospheric pressure increases the boiling point, as the water molecules need to overcome a greater external pressure to escape into the gaseous phase. Conversely, lower atmospheric pressure (like at high altitudes) lowers the boiling point.
-
Dissolved Substances: The presence of dissolved substances in water (like salt) can elevate the boiling point, a phenomenon known as boiling point elevation. This is because the dissolved particles interfere with the escape of water molecules from the liquid phase.
-
Impurities: Impurities in the water can also influence the boiling point and the overall heat transfer process.
Practical Applications and Real-World Examples
The endothermic nature of boiling water has numerous practical applications:
-
Cooking: Boiling water is fundamental to many cooking methods, from boiling pasta to steaming vegetables. The energy absorbed during boiling helps to cook food evenly.
-
Steam Power Generation: Power plants utilize the endothermic nature of boiling water to generate steam, which then drives turbines to produce electricity. The heat energy absorbed by the water is ultimately converted into mechanical and electrical energy.
-
Cooling Systems: Evaporation is an endothermic process closely related to boiling. The absorption of heat during evaporation is the basis of many cooling systems, such as sweat cooling the human body and evaporative coolers.
-
Industrial Processes: Many industrial processes rely on boiling water for cleaning, sterilization, and other applications. The heat absorption during boiling makes it an efficient method for these tasks.
Misconceptions and Clarifications
It's crucial to address common misconceptions surrounding the endothermic nature of boiling:
-
Temperature Increase After Boiling: While the temperature of boiling water remains constant at its boiling point (under constant pressure), the continued addition of heat energy drives the phase transition to steam. The temperature won't increase until all the liquid water has converted to steam.
-
Exothermic Condensation: The reverse process, condensation (steam turning back into liquid water), is exothermic because heat is released when the water molecules form intermolecular bonds. This is why steam can cause burns—it releases considerable heat upon condensation.
Conclusion: A Clear and Endothermic Process
In conclusion, boiling water is undeniably an endothermic process. The system absorbs heat energy from its surroundings to overcome the intermolecular forces holding water molecules together in the liquid state, driving the phase transition to steam. This fundamental concept plays a crucial role in various scientific fields and practical applications. Understanding the principles of heat transfer and phase changes is essential for grasping the complexities of the physical world. The high latent heat of vaporization and the influence of factors like atmospheric pressure and dissolved substances further emphasize the intricacies of this seemingly simple process.
Latest Posts
Latest Posts
-
What Is The Lowest Common Multiple Of 8 And 12
Mar 10, 2025
-
How Many Miles Is 3000 Km
Mar 10, 2025
-
How Many Seconds Is 3 Hours
Mar 10, 2025
-
1 5 Liters Is How Many Gallons
Mar 10, 2025
-
What Is The Hottest Color Star
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
