Is Carbon Dioxide A Pure Substance Or Mixture
Kalali
Mar 18, 2025 · 5 min read
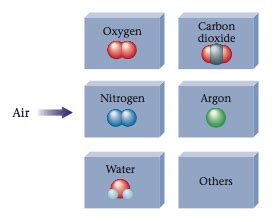
Table of Contents
Is Carbon Dioxide a Pure Substance or a Mixture? A Deep Dive into Chemical Composition
The question of whether carbon dioxide (CO2) is a pure substance or a mixture often arises in chemistry discussions. Understanding this requires a clear grasp of the definitions of pure substances and mixtures, and a closer look at the composition and behavior of CO2. This article will explore these aspects, providing a comprehensive answer and delving into related concepts.
Understanding Pure Substances and Mixtures
Before classifying CO2, let's define our terms:
Pure Substances
A pure substance is a form of matter that has a constant composition and properties throughout the sample. This means it's made up of only one type of atom or molecule. Pure substances can be further categorized into:
-
Elements: These are substances made up of only one type of atom, such as oxygen (O), iron (Fe), or gold (Au). They cannot be broken down into simpler substances by chemical means.
-
Compounds: These are substances made up of two or more different types of atoms chemically bonded together in fixed proportions. Examples include water (H₂O), table salt (NaCl), and carbon dioxide (CO₂). Compounds can be broken down into simpler substances through chemical reactions.
Mixtures
A mixture is a combination of two or more substances that are physically mixed but not chemically bonded. The substances retain their individual properties and can be separated by physical methods like filtration, distillation, or evaporation. Mixtures can be:
-
Homogeneous: These mixtures have a uniform composition throughout the sample. Examples include saltwater, air, and sugar dissolved in water.
-
Heterogeneous: These mixtures have a non-uniform composition; different components are visible. Examples include sand and water, oil and water, and a salad.
The Composition of Carbon Dioxide
Carbon dioxide (CO₂) is a chemical compound, not a mixture. It consists of one carbon atom (C) covalently bonded to two oxygen atoms (O). This bonding creates a distinct molecule with unique properties. No matter where you find CO₂, whether it's in the atmosphere, dissolved in soda, or produced during combustion, its molecular structure remains consistent: one carbon atom and two oxygen atoms. This fixed composition is the defining characteristic of a pure substance.
The Molecular Structure of CO2
The linear arrangement of atoms in CO₂ (O=C=O) is crucial to its properties. The strong double bonds between the carbon and oxygen atoms result in a relatively stable molecule. This stability contributes to CO₂'s behavior as a gas at standard temperature and pressure, and its ability to absorb and radiate infrared radiation, a key factor in the greenhouse effect.
Distinguishing CO2 from Mixtures
Let's contrast CO₂ with mixtures to reinforce its classification as a pure substance:
-
Air: Air is a homogeneous mixture of various gases, including nitrogen, oxygen, carbon dioxide, argon, and trace amounts of other gases. The proportion of these gases varies depending on location and altitude. CO₂, while present in air, is only one component of this complex mixture.
-
Soda Water: Soda water contains dissolved CO₂ in water. While it appears homogeneous, it's a mixture because the CO₂ can be separated from the water through techniques like boiling or depressurization. The CO₂ molecules themselves, however, remain pure CO₂ molecules.
-
Combustion Products: Burning fuels like wood or fossil fuels produces CO₂, along with other gases and particulates. This is a heterogeneous mixture. The CO₂ produced is still a pure substance, but it's part of a larger, complex mixture.
The Significance of Purity in Carbon Dioxide
The purity of CO₂ is critical in many applications:
-
Food and Beverage Industry: High-purity CO₂ is used in carbonated drinks to achieve consistent carbonation. Impurities can affect taste and quality.
-
Medical Applications: CO₂ is used in laser surgery and other medical procedures. Impurities could have adverse effects on patients.
-
Industrial Processes: CO₂ is employed in various industrial applications, including fire extinguishers, welding, and enhanced oil recovery. Purity is essential for safety and efficiency.
-
Scientific Research: Accurate measurements and experiments require high-purity CO₂ to avoid influencing results.
Common Misconceptions about CO2 Purity
Some misunderstandings about CO₂'s purity arise from its presence in mixtures:
-
Atmospheric CO₂: While atmospheric CO₂ is part of a mixture, the individual CO₂ molecules remain chemically pure. The focus is on the concentration of CO₂ within the mixture, not its chemical purity.
-
CO₂ from Different Sources: CO₂ produced from different sources (e.g., respiration, combustion, volcanic activity) may contain trace impurities, but the CO₂ molecule itself remains the same. These impurities are what differentiate the source of the CO₂, not the inherent purity of the CO₂ molecule.
Conclusion: Carbon Dioxide is a Pure Substance
In conclusion, carbon dioxide (CO₂) is a pure substance, specifically a compound. It's composed of carbon and oxygen atoms bonded in a fixed ratio (1:2). While it can be found in mixtures, such as air or soda water, this doesn't alter the chemical identity or purity of the CO₂ molecules themselves. The purity of CO₂ is paramount in various applications, highlighting the importance of understanding its chemical composition and behavior. The distinction between a pure substance and a mixture is crucial in chemistry, and CO₂ provides a clear example of a pure compound existing within complex mixtures. The consistent molecular structure of CO2, regardless of its source or surrounding environment, firmly establishes its classification as a pure substance. Understanding this fundamental concept is key to comprehending various chemical and environmental processes.
Latest Posts
Latest Posts
-
Temperate Deciduous Forest Oak Tree Adaptations
Mar 19, 2025
-
What Animals Can See Human Bioluminescence
Mar 19, 2025
-
Is Sour Taste A Physical Property
Mar 19, 2025
-
How Many Kilos Are 20 Pounds
Mar 19, 2025
-
11 Out Of 30 As A Percentage
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Carbon Dioxide A Pure Substance Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
