Is Cellular Respiration Endothermic Or Exothermic
Kalali
Mar 10, 2025 · 5 min read
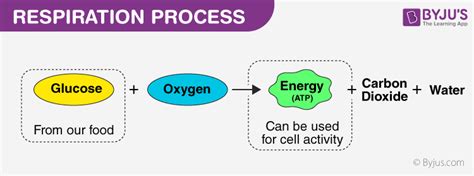
Table of Contents
Is Cellular Respiration Endothermic or Exothermic? Understanding Energy Flow in Living Organisms
Cellular respiration, the process by which cells break down glucose to produce ATP (adenosine triphosphate), the energy currency of life, is a fundamental process in all living organisms. A key question that arises in understanding this crucial metabolic pathway is whether it's endothermic or exothermic. The answer, while seemingly straightforward, requires a deeper dive into the thermodynamics of the reaction and its implications for life itself. This article will explore the intricacies of cellular respiration, definitively answering the question and clarifying common misconceptions.
Defining Endothermic and Exothermic Reactions
Before delving into the specifics of cellular respiration, let's establish a clear understanding of endothermic and exothermic processes. These terms describe the energy changes that occur during chemical reactions:
-
Exothermic Reactions: These reactions release energy into their surroundings. The products have lower energy than the reactants, and the excess energy is often released as heat. Think of burning wood – the chemical energy in the wood is converted into heat and light, warming the surroundings. The change in enthalpy (ΔH), a measure of heat change at constant pressure, is negative for exothermic reactions.
-
Endothermic Reactions: These reactions absorb energy from their surroundings. The products have higher energy than the reactants, requiring an input of energy to proceed. Melting ice is a good example; energy is absorbed from the surroundings to break the bonds holding the water molecules in the ice crystal structure. The change in enthalpy (ΔH) is positive for endothermic reactions.
Cellular Respiration: An Overview
Cellular respiration is a series of catabolic reactions that break down glucose, a simple sugar, in the presence of oxygen to produce ATP, carbon dioxide (CO2), and water (H2O). This process occurs in stages:
-
Glycolysis: This initial step takes place in the cytoplasm and breaks down glucose into two molecules of pyruvate. A small amount of ATP is produced.
-
Pyruvate Oxidation: Pyruvate is transported into the mitochondria, where it's converted into acetyl-CoA, releasing carbon dioxide.
-
Krebs Cycle (Citric Acid Cycle): Acetyl-CoA enters the Krebs cycle, a series of reactions that further oxidize the carbon atoms, releasing more carbon dioxide and generating high-energy electron carriers (NADH and FADH2). A small amount of ATP is also produced.
-
Oxidative Phosphorylation (Electron Transport Chain and Chemiosmosis): This is the most energy-yielding stage. Electrons from NADH and FADH2 are passed along a chain of protein complexes embedded in the inner mitochondrial membrane. This electron flow generates a proton gradient across the membrane, which drives the synthesis of a large amount of ATP through chemiosmosis. Oxygen acts as the final electron acceptor, forming water.
Cellular Respiration: Exothermic or Endothermic? The Definitive Answer
The overwhelming evidence demonstrates that cellular respiration is an exothermic process. This is because the energy stored in the chemical bonds of glucose is released during the breakdown process. The products, CO2 and H2O, have significantly lower energy content than the reactant, glucose. This energy difference is harnessed to produce ATP. The release of heat is evident in the fact that our bodies maintain a constant temperature, partially due to the heat generated by cellular respiration. The negative change in enthalpy (ΔH) confirms its exothermic nature.
Understanding the Energy Release
The energy released during cellular respiration is not simply dissipated as heat; it's carefully controlled and channeled into the production of ATP. This is crucial because ATP is the molecule that fuels virtually all cellular activities, from muscle contraction to protein synthesis. The controlled release of energy prevents the sudden, uncontrolled explosion of energy that would occur in combustion.
Misconceptions and Clarifications
Despite the clear exothermic nature of cellular respiration, some misconceptions persist:
-
Confusion with Activation Energy: Some may incorrectly interpret the initial energy investment required to start glycolysis as evidence of an endothermic reaction. While an initial energy input is needed to initiate glycolysis (activation energy), this is a small amount compared to the overall energy released during the subsequent steps. The net energy change is still significantly negative.
-
Focusing solely on ATP synthesis: The process of ATP synthesis itself can be considered slightly endothermic, requiring energy input to add a phosphate group to ADP. However, this energy input is far outweighed by the vast amount of energy released during the oxidation of glucose, making the overall process undeniably exothermic.
-
Ignoring the heat produced: The heat produced during cellular respiration is crucial for maintaining body temperature in many organisms. This heat generation is a clear indicator of an exothermic reaction.
The Importance of Oxygen
The presence of oxygen is critical for the efficiency of cellular respiration. Oxygen acts as the final electron acceptor in the electron transport chain. Without it, the electron transport chain would halt, significantly reducing ATP production. Anaerobic respiration, which occurs in the absence of oxygen, yields far less ATP than aerobic respiration.
The Role of Enzymes
Cellular respiration is a complex series of reactions that are catalyzed by enzymes. Enzymes act as biological catalysts, lowering the activation energy required for each step to proceed. This makes the reactions more efficient and ensures that energy is released in a controlled manner.
Implications for Life
The exothermic nature of cellular respiration is fundamental to life as we know it. The energy released is harnessed to power all the cellular processes necessary for survival, growth, and reproduction. This controlled release of energy ensures that organisms can maintain homeostasis and carry out their vital functions.
Conclusion: Cellular Respiration – A Highly Efficient Exothermic Process
In conclusion, cellular respiration is undoubtedly an exothermic process. The large net release of energy from the breakdown of glucose, evidenced by the negative change in enthalpy, the production of heat, and the generation of ATP, makes this unequivocal. Understanding the thermodynamics of cellular respiration is critical for comprehending the fundamental processes that drive life on Earth. The controlled release of energy through this exothermic process ensures that organisms can harness the energy stored in glucose to fuel their essential life functions, powering everything from basic metabolic activities to complex cognitive processes. The efficiency and regulation of this process are testaments to the intricate design of biological systems.
Latest Posts
Latest Posts
-
What Country Has The Most Birds
Mar 10, 2025
-
29 Out Of 30 As A Percentage
Mar 10, 2025
-
125 Cm In Feet And Inches
Mar 10, 2025
-
Is Melting Point A Physical Or Chemical Property
Mar 10, 2025
-
10 Is 20 Percent Of What
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is Cellular Respiration Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
