Is Cellular Respiration Exothermic Or Endothermic
Kalali
Mar 10, 2025 · 5 min read
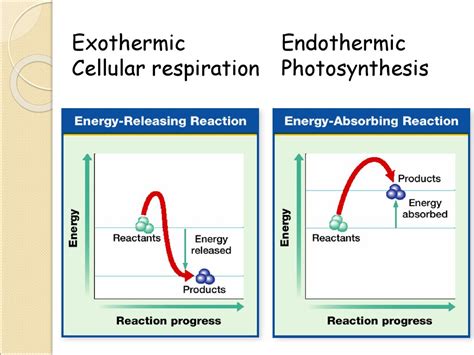
Table of Contents
Is Cellular Respiration Exothermic or Endothermic? A Deep Dive into Energy Production
Cellular respiration is a fundamental process in all living organisms, responsible for converting the chemical energy stored in food molecules into a readily usable form of energy: ATP (adenosine triphosphate). Understanding whether this process is exothermic or endothermic is crucial to grasping its role in life's processes. The simple answer is: cellular respiration is exothermic. However, let's delve deeper into the intricacies of this process to fully appreciate why.
Understanding Exothermic and Endothermic Reactions
Before examining cellular respiration, let's define our key terms. A chemical reaction is classified as exothermic if it releases energy into its surroundings. This energy is often released as heat, but it can also manifest as light or sound. Conversely, an endothermic reaction absorbs energy from its surroundings. This energy absorption usually results in a decrease in temperature of the surroundings.
Think of an exothermic reaction like burning wood: it produces heat and light, releasing energy stored within the wood's chemical bonds. An endothermic reaction is the opposite—like dissolving ammonium nitrate in water, which absorbs heat, leading to a drop in temperature.
The Exothermic Nature of Cellular Respiration
Cellular respiration is undeniably an exothermic process. This is because the chemical bonds in glucose (and other fuel molecules like fatty acids and amino acids) contain a significant amount of potential energy. During cellular respiration, these bonds are broken down, releasing this stored energy. A significant portion of this released energy is captured to synthesize ATP, the cell's primary energy currency. However, a considerable amount is also released as heat. This heat contributes to maintaining the body's temperature, especially crucial in warm-blooded animals (endotherms).
The overall reaction for cellular respiration, simplified, can be represented as:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy (ATP + Heat)
This equation shows that glucose (C₆H₁₂O₆) reacts with oxygen (O₂) to produce carbon dioxide (CO₂), water (H₂O), and energy in the form of ATP and heat. The energy released is a clear indication of the exothermic nature of the process.
Stages of Cellular Respiration: A Closer Look
To further solidify the exothermic nature of cellular respiration, let's examine its key stages:
1. Glycolysis: This initial stage occurs in the cytoplasm and involves the breakdown of glucose into pyruvate. While a small amount of ATP is produced directly (substrate-level phosphorylation), a net energy gain is achieved, and reducing power in the form of NADH is generated. While Glycolysis itself produces a small amount of ATP, it sets the stage for much more significant ATP production in subsequent stages. The overall process is exothermic, releasing some energy as heat.
2. Pyruvate Oxidation: Pyruvate, the product of glycolysis, enters the mitochondria and is converted into acetyl-CoA. This step also generates NADH, further contributing to the energy yield of respiration. Again, this stage is exothermic due to the release of energy during the conversion.
3. Krebs Cycle (Citric Acid Cycle): Within the mitochondrial matrix, the acetyl-CoA enters the Krebs cycle, a series of reactions that release carbon dioxide and generate ATP, NADH, and FADH₂ (another electron carrier). Each turn of the cycle produces a small amount of ATP directly and generates significant amounts of electron carriers crucial for the next phase. The oxidative decarboxylation reactions that occur here are exothermic, releasing energy in the form of ATP and reducing power.
4. Oxidative Phosphorylation (Electron Transport Chain and Chemiosmosis): This final stage is where the majority of ATP is produced. Electrons from NADH and FADH₂, generated in the previous stages, are passed along an electron transport chain embedded in the inner mitochondrial membrane. This electron flow generates a proton gradient across the membrane, which then drives ATP synthesis through chemiosmosis. The process involves a series of redox reactions, with each step releasing energy that contributes to the proton gradient and ultimately ATP synthesis. This highly efficient mechanism is also strongly exothermic. The large energy drop across the electron transport chain releases substantial heat.
The Importance of Heat Production in Cellular Respiration
The heat released during cellular respiration is not just a byproduct; it plays a vital role in maintaining homeostasis, particularly in endothermic animals. This heat helps regulate body temperature, enabling survival in various environments. The regulated release of heat prevents sudden temperature fluctuations and maintains a stable internal environment essential for enzymatic activity and other cellular processes.
Misconceptions and Clarifications
It's important to address potential misunderstandings. While some individual steps within cellular respiration might involve small energy inputs (e.g., the activation energy required to initiate certain reactions), the overall process is overwhelmingly exothermic. The energy released far surpasses the energy needed for activation of the individual steps.
Furthermore, while ATP synthesis itself requires some energy, this energy is dwarfed by the vast amount of energy released during electron transport and the other exothermic steps of cellular respiration. The net result remains a significant release of energy.
Conclusion: Cellular Respiration – A Masterpiece of Exothermic Energy Conversion
In summary, cellular respiration is demonstrably an exothermic process. The breakdown of glucose and other fuel molecules releases a significant amount of energy, a substantial portion of which is harnessed to synthesize ATP, the cell's energy currency. The remaining energy is released as heat, contributing to maintaining body temperature in endotherms and supporting various cellular functions. The meticulously regulated steps in cellular respiration ensure the efficient extraction and utilization of energy from food molecules, highlighting the remarkable efficiency and exothermic nature of this fundamental life process. Understanding this exothermic nature is fundamental to comprehending how life obtains and utilizes the energy required for its complex processes.
Latest Posts
Latest Posts
-
How Much Is 48 Oz Of Water
Jul 18, 2025
-
How Long Does It Take To Drive 2000 Miles
Jul 18, 2025
-
How Many Grams In A Teaspoon Of Cinnamon
Jul 18, 2025
-
How Long To Heat Water In Microwave
Jul 18, 2025
-
40 Oz Of Water Is How Many Cups
Jul 18, 2025
Related Post
Thank you for visiting our website which covers about Is Cellular Respiration Exothermic Or Endothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.