Is Fluorine A Cation Or Anion
Kalali
Mar 14, 2025 · 5 min read
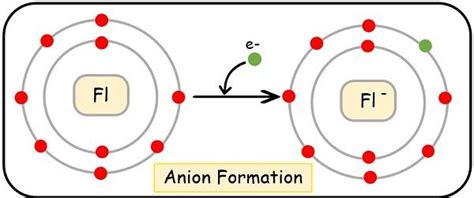
Table of Contents
Is Fluorine a Cation or an Anion? Understanding Fluorine's Ionic Nature
Fluorine, the most electronegative element on the periodic table, plays a crucial role in various chemical processes. A common question that arises when studying fluorine's chemical behavior is whether it forms a cation (a positively charged ion) or an anion (a negatively charged ion). This article delves deep into the intricacies of fluorine's ionic nature, exploring its electron configuration, electronegativity, and its propensity to form anions. We'll also examine exceptions and nuances in its reactivity to provide a comprehensive understanding of this fascinating element.
Understanding Ions: Cations and Anions
Before diving into the specifics of fluorine, let's refresh our understanding of ions. Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge.
-
Cations: These are positively charged ions, formed when an atom loses one or more electrons. Metals, with their relatively low electronegativity, tend to form cations. The loss of electrons allows them to achieve a more stable electron configuration, often resembling a noble gas.
-
Anions: These are negatively charged ions, formed when an atom gains one or more electrons. Nonmetals, with their high electronegativity, typically form anions. Gaining electrons completes their outermost electron shell, achieving a stable electron configuration.
Fluorine's Electron Configuration and Electronegativity
Fluorine (F) has an atomic number of 9, meaning it possesses 9 protons and 9 electrons in its neutral state. Its electron configuration is 1s²2s²2p⁵. This means its outermost shell (the 2p subshell) has only seven electrons, one electron short of a stable octet (eight electrons). This incomplete octet drives fluorine's chemical behavior.
Electronegativity is a crucial concept here. It measures an atom's ability to attract electrons towards itself within a chemical bond. Fluorine boasts the highest electronegativity of all elements. This exceptionally high electronegativity means fluorine has a strong tendency to attract electrons from other atoms.
Why Fluorine Forms An Anion (F⁻)
Given fluorine's high electronegativity and its one electron short of a stable octet, it's overwhelmingly more likely to gain an electron than to lose seven. Losing seven electrons would require an enormous amount of energy, making it highly improbable. Gaining a single electron is far more energetically favorable.
This electron gain results in the formation of a fluoride ion (F⁻), a stable anion with a complete octet (1s²2s²2p⁶), mirroring the electron configuration of the noble gas neon (Ne). The extra electron provides the stability that drives fluorine's reactivity.
In short: Fluorine almost exclusively forms anions (fluoride ions, F⁻) due to its incredibly high electronegativity and the energetic favorability of gaining a single electron to achieve a stable octet.
Chemical Reactions and the Formation of Fluoride Ions
Fluorine's tendency to form fluoride ions is evident in its numerous chemical reactions. It readily reacts with most other elements, often violently, to form ionic compounds. These reactions involve fluorine accepting an electron from another atom, creating a fluoride ion and a cation from the other element.
For example, the reaction between fluorine and sodium (Na) produces sodium fluoride (NaF):
2F₂ + 2Na → 2Na⁺ + 2F⁻
This reaction showcases fluorine's strong oxidizing power – its ability to gain electrons from other substances. The sodium atom readily loses its single valence electron to achieve a stable octet, forming a sodium cation (Na⁺). The fluorine atoms gain these electrons, forming fluoride ions (F⁻). The electrostatic attraction between the oppositely charged ions leads to the formation of the ionic compound, sodium fluoride.
Exceptions and Nuances: Understanding the Rare Cases
While the overwhelming majority of fluorine compounds involve fluoride anions, there are extremely rare and highly specialized circumstances where fluorine might exhibit different behavior. These are typically found in highly reactive or unusual chemical environments and are exceptions rather than the rule.
One such example involves compounds with highly electronegative elements. In some extremely specific scenarios, fluorine could theoretically form a weak covalent bond with an exceptionally electronegative element, effectively sharing electrons rather than completely gaining one. However, even in these instances, the resulting bond would still have significant ionic character due to fluorine's immense electronegativity.
The Importance of Fluoride Ions
The formation of fluoride ions (F⁻) is incredibly important in various applications and natural processes. The most widely known example is the use of fluoride in dental hygiene. Fluoride ions help strengthen tooth enamel, making teeth more resistant to decay.
Fluoride ions also find applications in various industrial processes, including:
-
Refrigerants: Certain fluorocarbons were previously used as refrigerants, although their impact on the ozone layer led to their phasing out.
-
Plastics: Fluorine-containing polymers are known for their high thermal and chemical stability, making them suitable for various applications.
-
Nuclear technology: Fluorine plays a role in certain nuclear fuel processing techniques.
Conclusion: Fluorine's Predominantly Anionic Nature
To reiterate, fluorine, with its exceptionally high electronegativity, overwhelmingly forms anions (fluoride ions, F⁻). The energetic favorability of gaining a single electron to achieve a stable octet significantly outweighs the possibility of losing seven electrons to form a cation. While exceedingly rare exceptions might exist under highly specific and extreme conditions, fluorine's anionic nature is fundamental to its chemistry and its significant roles in various applications and natural processes. The fluoride ion (F⁻) is a cornerstone of fluorine's influence on our world, from protecting our teeth to playing a role in industrial processes. Understanding its anionic behavior is crucial for grasping the broader implications of this vital element.
Latest Posts
Latest Posts
-
How Many Inches In 49 Cm
Mar 14, 2025
-
How Many Feet In 2 10 Of A Mile
Mar 14, 2025
-
What Is 7 3 As A Decimal
Mar 14, 2025
-
55 Degrees Fahrenheit Is What In Celsius
Mar 14, 2025
-
How Many Feet Are In 2 10 Of A Mile
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Is Fluorine A Cation Or Anion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
