Is Frying An Egg A Chemical Change Or Physical
Kalali
Mar 14, 2025 · 6 min read
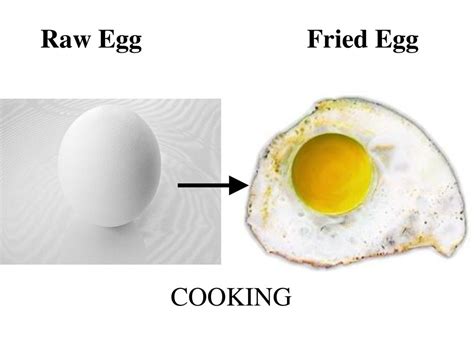
Table of Contents
Is Frying an Egg a Chemical Change or a Physical Change? A Deep Dive into Culinary Chemistry
The seemingly simple act of frying an egg sparks a fascinating debate in the world of chemistry: is it a physical change or a chemical change? While the answer might seem obvious at first glance, a closer examination reveals a complex interplay of physical and chemical processes. This article will delve into the science behind cooking an egg, exploring the transformations involved and clarifying the distinction between physical and chemical changes.
Understanding the Basics: Physical vs. Chemical Changes
Before we tackle the egg-frying conundrum, let's establish a clear understanding of the difference between physical and chemical changes. A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think of melting ice – it changes from a solid to a liquid, but it's still H₂O. The molecules themselves remain unchanged.
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Burning wood is a classic example; the wood's chemical structure breaks down, forming ashes, smoke, and gases. The resulting products are fundamentally different from the original wood.
The Egg's Composition: A Complex Mixture
To understand the changes during egg frying, we need to know what an egg is made of. An egg is a remarkably complex mixture, primarily composed of:
- Water: A significant component, contributing to the egg's fluidity and texture.
- Proteins: The most important component for our discussion. Egg white (albumen) is primarily composed of albumen proteins, while the yolk contains lipoproteins (proteins bound to lipids). These proteins are long chains of amino acids, folded into intricate 3D structures.
- Lipids (Fats): Concentrated mainly in the yolk, these contribute to the egg's richness and creamy texture.
- Carbohydrates: Present in smaller amounts, mainly in the form of sugars.
- Minerals and Vitamins: Essential nutrients contributing to the egg's nutritional value.
The Frying Process: A Cascade of Transformations
When you fry an egg, several processes occur simultaneously, making it a complex blend of both physical and chemical changes.
Physical Changes During Egg Frying
- Heat Transfer: The primary physical process is heat transfer from the pan to the egg. This causes an increase in the egg's temperature.
- Water Evaporation: As the egg heats, water within the egg white and yolk evaporates, leading to a reduction in volume and a change in texture. This is purely a physical change—the water remains water, only changing its state from liquid to gas.
- Protein Denaturation (Initial Stage): Initially, the heat causes the proteins to unfold. This unfolding, or denaturation, is a physical change; the amino acid sequence remains unchanged. Think of it as a neatly folded sweater being stretched out – it's still the same sweater, but it's lost its shape. This is primarily responsible for the egg white becoming opaque.
Chemical Changes During Egg Frying
- Protein Coagulation: This is the key chemical change. As the denatured proteins continue to heat, they form new bonds with each other, creating a tangled network. This process is called coagulation. It's a chemical change because new bonds are formed between the protein molecules, resulting in a significantly different structure and properties. The resulting solid structure is no longer the same as the original soluble protein.
- Maillard Reaction: This is a complex chemical reaction between amino acids and reducing sugars (carbohydrates) at high temperatures. It's responsible for the browning and characteristic flavor development of the egg's surface. The Maillard reaction creates hundreds of new flavor compounds, fundamentally altering the egg's chemical composition.
- Lipid Oxidation: The yolk's lipids can undergo oxidation, especially at high temperatures. This is a chemical change leading to rancidity and changes in flavor and smell. The oxidation process involves the reaction of lipids with oxygen, forming different chemical compounds.
Why it's Primarily a Chemical Change
While several physical changes occur during egg frying, the defining transformation is the irreversible chemical change brought about by protein coagulation and the Maillard reaction. These processes create new substances with different properties than the original egg components. You cannot reverse the process and regain the original liquid egg white and runny yolk. The egg has undergone a fundamental alteration in its chemical structure.
Distinguishing between denaturation and coagulation: a subtle but crucial difference.
It's important to note that while protein denaturation is often initially described as a physical change (unfolding), the subsequent coagulation which forms cross-links between the denatured proteins is decisively a chemical change. The initial unfolding makes the later formation of these new bonds possible but it is the formation of these new bonds which firmly places the process within the realm of chemical change. The initial denaturation is therefore a precursor to the irreversible chemical change of coagulation.
Exploring further aspects: The impact of frying techniques</h2>
The extent of the chemical changes during frying also depends on various factors, including:
- Temperature: Higher temperatures accelerate the Maillard reaction and lipid oxidation, leading to a browner, crispier egg with a more intense flavor (but possibly with increased rancidity). Lower temperatures result in a gentler cooking process with less browning and different flavor profiles.
- Cooking Time: Longer cooking times increase the extent of protein coagulation and the Maillard reaction, resulting in a firmer, drier egg.
- Presence of Fat: Frying in oil or butter affects both the physical and chemical changes. The fat acts as a heat transfer medium and can also contribute to the Maillard reaction and lipid oxidation.
Conclusion: A Culinary Chemical Reaction
In summary, frying an egg is predominantly a chemical change. While physical changes like water evaporation and initial protein unfolding contribute to the process, it's the irreversible chemical changes, particularly protein coagulation and the Maillard reaction, that fundamentally alter the egg's composition and properties. The fried egg is a chemically different substance compared to the raw egg. Understanding these complex chemical and physical processes allows us to appreciate the science behind a seemingly simple cooking technique and to explore the culinary possibilities by controlling the parameters of the cooking process.
Keywords for SEO Optimization
- Frying an egg
- Chemical change
- Physical change
- Protein denaturation
- Protein coagulation
- Maillard reaction
- Lipid oxidation
- Culinary chemistry
- Egg composition
- Cooking science
Semantic Keywords for SEO Optimization
- Is cooking an egg a chemical or physical change?
- What happens to proteins when you fry an egg?
- Science of cooking eggs
- Chemical reactions in cooking
- How frying changes the structure of an egg
- The chemistry of food
This expanded article incorporates a range of SEO techniques, including keyword optimization, semantic keyword inclusion, and a structured format to enhance readability and search engine ranking. The focus is on providing a comprehensive and engaging explanation of the science behind frying an egg, catering to both culinary enthusiasts and science students.
Latest Posts
Latest Posts
-
How Long Does It Take To Drive 2000 Miles
Jul 18, 2025
-
How Many Grams In A Teaspoon Of Cinnamon
Jul 18, 2025
-
How Long To Heat Water In Microwave
Jul 18, 2025
-
40 Oz Of Water Is How Many Cups
Jul 18, 2025
-
How Many Eighths In A Quarter Pound
Jul 18, 2025
Related Post
Thank you for visiting our website which covers about Is Frying An Egg A Chemical Change Or Physical . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.