Is Frying An Egg A Physical Change
Kalali
Mar 19, 2025 · 6 min read
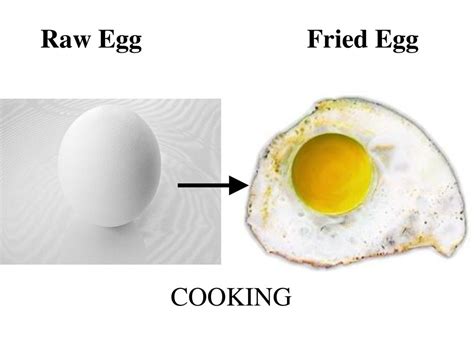
Table of Contents
Is Frying an Egg a Physical Change? A Deep Dive into Culinary Chemistry
The seemingly simple act of frying an egg is a surprisingly complex process, a miniature theater of chemical and physical transformations. While it might appear at first glance to be merely a physical change—a change in shape and state—a closer examination reveals a fascinating interplay of chemical reactions that fundamentally alter the egg's composition. This article will explore the science behind frying an egg, examining whether it constitutes a physical change, a chemical change, or a combination of both. We'll delve into the specific transformations occurring within the egg white and yolk, considering factors like temperature, time, and the presence of fat.
Understanding Physical vs. Chemical Changes
Before diving into the specifics of frying an egg, let's establish a clear definition of physical and chemical changes.
Physical changes alter the form or appearance of a substance without changing its chemical composition. Examples include melting ice (water changes state but remains H₂O), dissolving sugar in water (sugar particles disperse but retain their chemical identity), or tearing paper (the paper's shape changes, but it remains cellulose). Crucially, physical changes are reversible in many cases.
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Examples include burning wood (cellulose reacts with oxygen to produce carbon dioxide and water), rusting iron (iron reacts with oxygen to form iron oxide), or baking a cake (ingredients undergo chemical reactions to form a complex new structure). Chemical changes are generally irreversible.
The Egg's Composition: A Starting Point
To understand the changes during frying, we need to appreciate the egg's constituents. An egg consists primarily of:
- Water: A significant portion of both the white (albumen) and yolk.
- Proteins: These are complex molecules, crucial for the egg's structure and functionality. The white is rich in albumen proteins (like ovalbumin, conalbumin, ovomucoid), while the yolk contains lipoproteins and phospholipids alongside proteins like livetin and phosvitin.
- Lipids (fats): Concentrated in the yolk, these provide richness and creaminess.
- Carbohydrates: Present in smaller amounts.
- Minerals and Vitamins: Various nutrients contributing to the egg's nutritional value.
Frying the Egg: A Step-by-Step Analysis
Let's break down the process of frying an egg to analyze the transformations:
1. Heat Application: Initial Stages
As the pan heats, the water in the egg white begins to heat up. This is a physical change. The water molecules gain kinetic energy, increasing their movement.
2. Denaturation of Proteins: The White's Transformation
As the temperature reaches around 60°C (140°F), a crucial chemical change begins: protein denaturation. Heat disrupts the weak bonds (hydrogen bonds, disulfide bonds) holding the protein molecules in their original folded shapes. This causes the proteins to unfold and aggregate, forming a network that solidifies the egg white. This is an irreversible chemical change. The transparent, liquid egg white becomes opaque and white.
The process is not uniform. Different proteins denature at slightly different temperatures, leading to the characteristic texture and appearance of the cooked egg white. The edges solidify first, followed by the interior.
3. Coagulation: Setting the White
The aggregated proteins form a gel-like structure, a process called coagulation. This is a further consequence of protein denaturation, leading to the characteristic firm texture of the cooked egg white. This is primarily a chemical change, resulting in a new structure and properties of the protein network.
4. Heat Transfer and the Yolk's Fate
Heat continues to transfer to the yolk. The yolk, being richer in lipids, requires a higher temperature to solidify. Water within the yolk also evaporates, contributing to slight shrinkage.
5. Yolk Denaturation and Coagulation
The proteins in the yolk also undergo denaturation and coagulation, but at a higher temperature than the white. The yolk proteins are different from those in the white, leading to a different texture and consistency. The yolk solidifies more slowly, resulting in a creamier, less firm consistency compared to the white. This is a further irreversible chemical change.
6. Maillard Reaction and Browning
If the temperature gets high enough, the Maillard reaction can occur. This is a complex series of chemical reactions between amino acids and reducing sugars in the egg, resulting in browning and the development of characteristic flavors and aromas. This is a clear chemical change, producing new compounds not present in the raw egg.
7. Lipid Changes
The lipids in the yolk, particularly if the egg is fried in oil, undergo changes as well. Some lipids may oxidize, contributing to flavor and aroma changes. While the lipid structure itself might not be fundamentally altered, the oxidation reaction is a clear chemical change.
The Verdict: Physical and Chemical Changes Combined
Frying an egg is not solely a physical change. While changes in shape and state (liquid to solid) are undeniably physical transformations, the fundamental changes in the egg's protein structure, the Maillard reaction, and lipid oxidation all represent irreversible chemical changes.
The protein denaturation and coagulation are arguably the most significant chemical transformations. These processes are driven by heat and lead to substantial changes in the egg's physical properties, including texture, appearance, and digestibility. The irreversible nature of these changes firmly places the process beyond a simple physical transformation.
Factors Influencing the Process
Several factors influence the outcome of frying an egg:
- Temperature: Higher temperatures accelerate denaturation and coagulation, potentially leading to a drier, tougher egg. Lower temperatures lead to a softer, runnier egg.
- Time: Longer frying times lead to more extensive protein denaturation and coagulation, resulting in a more solid egg.
- Cooking Method: Different cooking methods, like scrambling, poaching, or sunny-side up, impact the degree and distribution of protein denaturation and coagulation.
- Fat Type: The type of fat used for frying can influence the flavor and browning due to differences in lipid composition and oxidation properties.
Conclusion: A Complex Culinary Transformation
The seemingly simple act of frying an egg is a fascinating blend of physical and chemical changes. While the overall change in state is physical, the underlying molecular transformations, specifically protein denaturation, coagulation, the Maillard reaction, and lipid oxidation, are undeniably chemical. The irreversible nature of these chemical processes highlights that frying an egg is far more than a simple physical alteration; it's a complex culinary process steeped in chemistry. The exact balance between physical and chemical changes depends on numerous factors, making each fried egg a unique product of its own preparation. This intricate interplay of science and art is what makes cooking such an engaging and enriching experience.
Latest Posts
Latest Posts
-
How To Write 2 1 2 As A Decimal
Mar 19, 2025
-
In What Ph Range Is Hair Skin And Nails
Mar 19, 2025
-
What Is The Molar Mass Of Fecl3
Mar 19, 2025
-
How Many Feet In 192 Inches
Mar 19, 2025
-
1 3 4 In To Mm
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Frying An Egg A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
