Is Gasoline A Homogeneous Or Heterogeneous Mixture
Kalali
Mar 11, 2025 · 5 min read
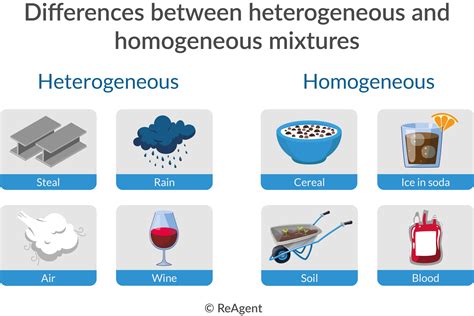
Table of Contents
Is Gasoline a Homogeneous or Heterogeneous Mixture? A Deep Dive
Gasoline, the lifeblood of countless vehicles worldwide, is more than just a simple substance. Understanding its true nature requires delving into the fascinating world of chemistry and exploring the concepts of homogeneous and heterogeneous mixtures. This comprehensive article will explore the composition of gasoline, examine the arguments for classifying it as either homogeneous or heterogeneous, and ultimately provide a definitive answer based on scientific understanding.
Understanding Homogeneous and Heterogeneous Mixtures
Before we delve into the specifics of gasoline, let's clarify the fundamental difference between homogeneous and heterogeneous mixtures:
Homogeneous Mixture: A homogeneous mixture is a type of mixture where the composition is uniform throughout. At a microscopic level, the individual components are indistinguishable, creating a visually uniform appearance. Examples include saltwater, air, and many metal alloys.
Heterogeneous Mixture: A heterogeneous mixture, on the other hand, has a non-uniform composition. Different components are visible and can be easily separated. Examples include sand and water, oil and water, and a salad. The key differentiator is the lack of uniform distribution of components.
The Complex Composition of Gasoline
Gasoline isn't a single compound; it's a complex mixture of hydrocarbons – molecules composed primarily of carbon and hydrogen atoms. These hydrocarbons vary significantly in their molecular structure and size. The specific composition of gasoline varies depending on several factors, including:
- Crude Oil Source: Different crude oil sources contain varying proportions of different hydrocarbons.
- Refining Process: The refining process significantly impacts the final composition of gasoline. This includes processes like fractional distillation, which separates crude oil into different fractions based on boiling points. Additives are also introduced during refining.
- Season: The composition of gasoline may be adjusted seasonally to optimize performance in different weather conditions. For example, winter blends have a higher volatility to aid cold-weather starting.
Key Components of Gasoline
Gasoline primarily consists of a complex blend of:
-
Alkanes (Paraffins): These are straight-chain or branched-chain saturated hydrocarbons. They are a major constituent of gasoline, contributing to its energy content. Examples include butane, pentane, hexane, and octane.
-
Alkenes (Olefins): These are unsaturated hydrocarbons containing at least one carbon-carbon double bond. They contribute to gasoline's octane rating and volatility. Examples include butene, pentene, and hexene.
-
Aromatics: These are hydrocarbons containing a benzene ring structure. They contribute to gasoline's octane rating and energy density. Examples include benzene, toluene, and xylene.
-
Additives: Numerous additives are incorporated into gasoline to improve its performance and stability. These additives include detergents, antioxidants, corrosion inhibitors, and octane boosters.
The Argument for Gasoline as a Homogeneous Mixture
At a macroscopic level, gasoline appears to be a uniform liquid. It doesn't exhibit distinct layers or visibly separated components. When viewed with the naked eye, or even with a simple magnifying glass, the mixture appears consistent throughout. This visual uniformity is a strong argument for classifying gasoline as homogeneous.
Furthermore, the components of gasoline are thoroughly mixed during the refining process. The mixing is so complete that the individual hydrocarbons are dispersed at a molecular level. This creates a solution where the concentration of each component is relatively uniform throughout the entire volume of gasoline. This molecular-level uniformity further supports the classification as a homogeneous mixture.
Finally, the physical properties of gasoline, such as density and boiling point, are relatively consistent throughout the sample. These consistent properties are characteristic of a homogeneous mixture where the properties of the individual components are effectively averaged out.
The Argument for Gasoline as a Heterogeneous Mixture
While the macroscopic uniformity of gasoline makes a compelling case for homogeneity, a closer examination reveals nuances that could lead one to argue for its heterogeneous nature.
The argument rests on the presence of additives and the inherent variability in the concentration of different hydrocarbons. While the mixing process aims for uniformity, microscopic variations in the distribution of these components might exist. These variations, though likely minuscule, could technically classify gasoline as heterogeneous at a very high level of scrutiny. Advanced analytical techniques, such as chromatography, can indeed reveal subtle differences in composition across different samples of gasoline.
Moreover, different batches of gasoline, even from the same refinery, can have slightly varying compositions due to the inherent fluctuations in the refining process and the raw materials used. This inherent variability, though subtle in terms of macroscopic properties, contributes to the argument for heterogeneity.
Resolving the Debate: A Practical Perspective
The debate of whether gasoline is homogeneous or heterogeneous ultimately hinges on the scale of observation. At the macroscopic level – the scale of everyday observation and usage – gasoline behaves as a homogeneous mixture. Its uniform appearance, consistent properties, and even distribution of components justify this classification for all practical purposes.
However, at a microscopic level, or when considering the extremely subtle variations in composition across different samples, one could argue for heterogeneity. These variations are typically negligible for most practical applications and do not impact the performance or usability of gasoline.
Therefore, while technically minute variations in composition could classify gasoline as heterogeneous at a highly refined analytical level, it is more practical and accurate to classify gasoline as a homogeneous mixture for all intents and purposes. This classification accurately reflects its behavior in most applications and aligns with common scientific understanding of its properties.
Conclusion: Gasoline – A Homogeneous Mixture in Practice
The journey into the composition of gasoline has highlighted the complexities of classifying mixtures. While the subtleties of its composition raise valid points about heterogeneity at a microscopic level, the overwhelming evidence points towards gasoline's practical classification as a homogeneous mixture. Its uniform appearance, consistent properties, and widespread application as a uniform fuel solidify this categorization. Understanding this classification is essential for comprehending its properties, its use in various applications, and its role in the global energy landscape.
Latest Posts
Latest Posts
-
What Is The Average Iq For 14 Year Old
Jul 14, 2025
-
Is Banana Pudding A Homo Or Heteronegous Mixture
Jul 14, 2025
-
What Year Were You Born In If You Are 15
Jul 14, 2025
-
How Much Is A Peck Of Peppers
Jul 14, 2025
-
How Does The Monster Try To Gain Control Of Victor
Jul 14, 2025
Related Post
Thank you for visiting our website which covers about Is Gasoline A Homogeneous Or Heterogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.