Is Lithium A Solid Liquid Or Gas
Kalali
Mar 21, 2025 · 6 min read
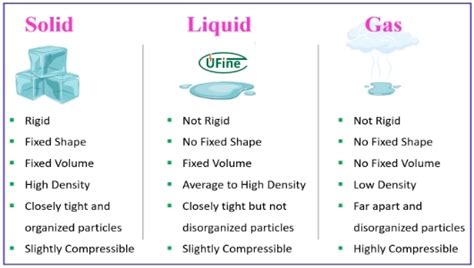
Table of Contents
Is Lithium a Solid, Liquid, or Gas? Understanding Lithium's Properties
Lithium, a fascinating and crucial element in modern technology, often sparks curiosity about its physical state. The simple question, "Is lithium a solid, liquid, or gas?" deserves a more nuanced answer than a single word. This comprehensive guide will delve into the properties of lithium, exploring its phase transitions, applications, and the factors influencing its state at various temperatures and pressures.
Understanding the Phases of Matter
Before examining lithium specifically, let's briefly review the three fundamental phases of matter: solid, liquid, and gas. These phases are characterized by the arrangement and movement of atoms or molecules:
-
Solid: Atoms or molecules in a solid are tightly packed in a regular, ordered structure. They vibrate in place but do not move freely. Solids have a definite shape and volume.
-
Liquid: Atoms or molecules in a liquid are close together but not in a fixed arrangement. They can move past each other, giving liquids a definite volume but an indefinite shape (they take the shape of their container).
-
Gas: Atoms or molecules in a gas are widely separated and move randomly at high speeds. Gases have neither a definite shape nor a definite volume, expanding to fill their container.
Lithium: A Solid at Room Temperature
Under standard conditions (room temperature and atmospheric pressure), lithium is a solid. This is crucial to understand because its solid nature dictates its many applications. Its silvery-white metallic appearance is characteristic of its solid crystalline structure. This structure, a body-centered cubic lattice, contributes to its properties like conductivity and reactivity.
The Importance of Temperature and Pressure
The state of matter is significantly influenced by temperature and pressure. While lithium is a solid at room temperature, increasing the temperature will eventually cause it to transition to a liquid, and further increases will lead to a gaseous state. Similarly, extreme pressure can also alter its phase. However, these transitions require considerably high temperatures and pressures, making the solid state the most relevant for most practical purposes.
Lithium's Melting and Boiling Points: A Closer Look
The transition between phases is defined by specific temperatures:
-
Melting Point: The temperature at which a solid transforms into a liquid. For lithium, the melting point is relatively low at 180.5 °C (357 °F). This relatively low melting point makes it easier to process and handle in certain applications compared to other metals with much higher melting points.
-
Boiling Point: The temperature at which a liquid transforms into a gas. Lithium's boiling point is significantly higher, at 1342 °C (2448 °F). This high boiling point demonstrates the strong metallic bonds within the lithium structure, requiring substantial energy to break them and transition to the gaseous phase.
These relatively low melting point and high boiling point, compared to some other alkali metals, reflect the unique atomic structure and bonding characteristics of lithium.
Applications of Solid Lithium: A Versatile Element
The fact that lithium is a solid at room temperature is paramount to its wide-ranging applications. Its unique properties make it essential in diverse fields:
1. Batteries: Powering the Modern World
Perhaps the most well-known application of lithium is in lithium-ion batteries. The solid lithium metal's electrochemical properties are crucial for the functionality of these rechargeable batteries, which power everything from smartphones and laptops to electric vehicles and grid-scale energy storage systems. The solid state of lithium within these batteries ensures stability and efficiency. Researchers continue to explore ways to improve the performance of lithium-ion batteries, including the exploration of solid-state lithium batteries which offer increased safety and energy density compared to the conventional liquid electrolyte lithium-ion batteries.
2. Lubricants: Reducing Friction
Lithium's solid-state properties are also leveraged in greases and lubricants. Lithium-based greases are known for their excellent thermal stability, water resistance, and ability to withstand high pressures. These properties make them suitable for a wide range of applications, from automotive components to industrial machinery, where reducing friction and wear is critical. The solid lithium compounds within these greases contribute to their effectiveness.
3. Ceramics and Glass: Enhancing Materials
Lithium compounds are used in the manufacturing of ceramics and glass. The addition of lithium enhances the properties of these materials, improving their durability, strength, and thermal resistance. This results in lighter, stronger, and more heat-resistant products across various industries, including construction and aerospace. This application further illustrates the role of lithium compounds, even when lithium itself isn't in its elemental form.
4. Medicine and Pharmaceuticals: Therapeutic Applications
Lithium salts are also employed in medicine, particularly in the treatment of bipolar disorder. Lithium's role in regulating mood stabilizers is a testament to its unique chemical properties that impact biological systems. This application, however, utilizes lithium in its ionic form (lithium salts), rather than the elemental lithium metal.
5. Nuclear Reactors: Specialized Applications
Lithium isotopes, such as lithium-6, have a crucial role in nuclear fusion reactors. These isotopes are utilized as neutron absorbers and multipliers in controlled fusion reactions. This specialized application highlights the unique nuclear properties of specific lithium isotopes which are not directly connected to the solid/liquid/gas question.
Exploring Lithium's Behavior Under Extreme Conditions
While lithium is solid under normal conditions, its behavior under extreme conditions (high temperatures and pressures) is fascinating:
-
High Temperatures: As mentioned, exceeding lithium's melting point (180.5 °C) transforms it into a liquid. Further increases in temperature lead to the gaseous phase, with its atoms moving freely. These high-temperature states are relevant in specialized industrial processes, though less common than its solid-state applications.
-
High Pressures: Applying extreme pressure can alter lithium's crystal structure and even induce phase transitions to novel, high-pressure phases. This is a field of ongoing research, exploring the behavior of materials under extreme conditions, pushing the boundaries of our understanding of material science.
Conclusion: The Solid State Dominates
In conclusion, the simple answer to the question "Is lithium a solid, liquid, or gas?" is: lithium is a solid at room temperature and atmospheric pressure. This solid state is fundamental to its extensive use in batteries, lubricants, ceramics, medicine, and other applications. While it can exist in liquid and gaseous phases under extreme conditions, its solid-state properties are the primary driver of its significance in modern technology and various industries. Understanding these phase transitions and the unique properties of lithium across these phases is crucial for advancing its applications and further harnessing its potential. Further research into the behavior of lithium under extreme conditions continues to uncover its versatile nature and possibilities.
Latest Posts
Latest Posts
-
38 Inches Is How Many Feet
Mar 21, 2025
-
Is Baking Cookies A Chemical Or Physical Change
Mar 21, 2025
-
24 Oz Is How Many Cups
Mar 21, 2025
-
What Percent Of 75 Is 15
Mar 21, 2025
-
What Is 118 Inches In Feet
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Is Lithium A Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
