Is Magnesium A Metal Metalloid Or Nonmetal
Kalali
Mar 14, 2025 · 6 min read
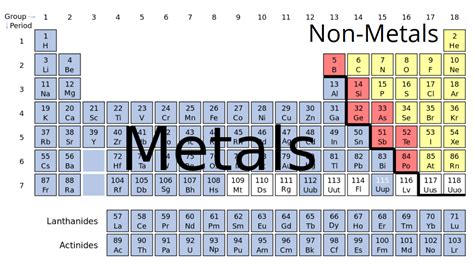
Table of Contents
Is Magnesium a Metal, Metalloid, or Nonmetal? A Comprehensive Exploration
Magnesium, a silvery-white element ubiquitous in the Earth's crust and essential for human life, often sparks the question: is it a metal, metalloid, or nonmetal? The answer, unequivocally, is metal. However, understanding why requires delving into the fascinating world of elemental properties and the periodic table's organization. This article will explore magnesium's characteristics, comparing them to metalloids and nonmetals, to conclusively establish its metallic nature and delve into its significant applications.
Understanding the Classification of Elements
Before diving into the specifics of magnesium, let's establish a foundational understanding of how elements are categorized. The periodic table arranges elements based on their atomic structure and resulting properties. This organization allows us to predict an element's behavior and reactivity. The three main categories are:
1. Metals
Metals typically exhibit:
- High electrical conductivity: They readily conduct electricity due to the ease with which electrons move through their structure.
- High thermal conductivity: They efficiently transfer heat.
- Malleability and ductility: They can be hammered into sheets (malleability) and drawn into wires (ductility) without breaking.
- Metallic luster: They possess a characteristic shiny appearance.
- Low ionization energy: They readily lose electrons to form positive ions (cations).
2. Metalloids (Semimetals)
Metalloids occupy a fascinating middle ground, exhibiting properties of both metals and nonmetals. Their characteristics are often intermediate and can vary depending on conditions. Key features include:
- Variable electrical conductivity: Their conductivity can change with temperature or other factors, leading to their use in semiconductors.
- Variable thermal conductivity: Similar to electrical conductivity, their thermal conductivity isn't consistently high or low.
- Brittle nature: Unlike metals, they tend to be brittle and don't exhibit significant malleability or ductility.
- Semiconductor properties: This is a defining characteristic, making them crucial in electronics.
3. Nonmetals
Nonmetals, in contrast to metals, generally display:
- Low electrical conductivity: They are poor conductors of electricity.
- Low thermal conductivity: They are poor conductors of heat.
- Brittle nature: They are usually brittle solids.
- Lack of metallic luster: They lack the characteristic shine of metals.
- High ionization energy: They tend to gain electrons to form negative ions (anions).
Magnesium's Metallic Properties: A Detailed Look
Now, let's specifically examine magnesium's properties in light of the classifications above. Magnesium's position on the periodic table, in Group 2 (alkaline earth metals), already strongly suggests its metallic nature. Let's analyze its key properties:
1. Electrical Conductivity
Magnesium is an excellent conductor of electricity. This stems from the ease with which its valence electrons (the outermost electrons) can move freely within the metallic lattice structure. This free electron movement is characteristic of metals and enables their ability to readily transmit electrical current.
2. Thermal Conductivity
Similarly, magnesium boasts high thermal conductivity. The freely moving electrons efficiently transfer thermal energy throughout the material, making it an effective conductor of heat. This property finds applications in various heat transfer systems.
3. Malleability and Ductility
Although not as malleable or ductile as some other metals like gold or copper, magnesium is still capable of being shaped and formed. It can be rolled into sheets and extruded into wires, albeit with more effort than some of its more malleable metallic counterparts. This property is still characteristically metallic.
4. Metallic Luster
Magnesium possesses a distinctive silvery-white metallic luster. This shiny appearance is a hallmark of metals and arises from the interaction of light with the free electrons in the metallic lattice.
5. Low Ionization Energy
Magnesium readily loses its two valence electrons to achieve a stable electron configuration. This relatively low ionization energy is a key indicator of its metallic nature and its tendency to form positive ions (Mg²⁺) in chemical reactions. This ability to readily lose electrons contributes to its reactivity and its role in various chemical processes.
6. Other Metallic Characteristics
Magnesium also demonstrates other metallic characteristics, such as its crystalline structure, its opacity (it doesn't transmit light), and its ability to form alloys with other metals. These features collectively reinforce its classification as a metal.
Comparing Magnesium to Metalloids and Nonmetals
To further solidify magnesium's classification as a metal, let's compare it to metalloids and nonmetals:
Magnesium vs. Metalloids
The key differentiator lies in conductivity. Metalloids exhibit semi-conductivity – their electrical conductivity is intermediate and often dependent on external factors such as temperature. Magnesium, however, is a highly conductive metal, demonstrating a consistent and robust ability to conduct electricity. Its malleability and ductility also stand in stark contrast to the brittle nature of metalloids. Furthermore, magnesium's distinct metallic luster separates it from the often dull appearance of metalloids.
Magnesium vs. Nonmetals
The contrast between magnesium and nonmetals is even more pronounced. Nonmetals are typically poor conductors of both electricity and heat, possessing high ionization energies. Magnesium’s high conductivity, low ionization energy, metallic luster, and malleability sharply contrast with these nonmetallic properties. Nonmetals often exist as gases or brittle solids at room temperature, another significant difference from magnesium's metallic solid state.
Applications of Magnesium's Metallic Properties
Magnesium's unique combination of lightness, strength, and reactivity makes it invaluable in a wide range of applications:
- Alloying agent: Magnesium's addition to aluminum alloys enhances their strength and reduces their weight, making them crucial in aerospace and automotive industries.
- Structural materials: Its lightness and strength are exploited in various structural applications where weight reduction is critical, such as in laptops, cameras, and other portable electronics.
- Reducing agent in metallurgy: Magnesium's high reactivity allows its use as a reducing agent in extracting other metals from their ores.
- Sacrificial anodes: Magnesium's susceptibility to corrosion is utilized in sacrificial anodes to protect other metals from corrosion.
- Fireworks and flares: Magnesium's bright white flame when burned contributes to its use in pyrotechnics.
- Biological importance: Magnesium is essential for numerous biological processes, acting as a cofactor in enzymatic reactions and playing a critical role in muscle function and nerve transmission.
Conclusion: Magnesium is a Metal
Based on its comprehensive set of properties, magnesium unequivocally fits the definition of a metal. Its high electrical and thermal conductivity, malleability, ductility, metallic luster, low ionization energy, and numerous applications that directly benefit from these metallic properties leave no doubt. Any comparison to metalloids or nonmetals reveals significant differences, further reinforcing its classification as a metal and highlighting its vital role in various technological and biological systems. Its unique blend of properties makes it a valuable element, crucial for numerous applications, from lightweight alloys to biological processes. The metallic nature of magnesium isn't just a theoretical classification; it is a fundamental aspect that underpins its significance and utility in the modern world.
Latest Posts
Latest Posts
-
How Do You Spell To In French
Jul 14, 2025
-
Did Faith Hill Have An Affair With Alan Jackson
Jul 14, 2025
-
What Is The Surface Area Of The Cube Below
Jul 14, 2025
-
How Many Grams Is Quarter Pound Of Weed
Jul 14, 2025
-
7am To 11pm Is How Many Hours
Jul 14, 2025
Related Post
Thank you for visiting our website which covers about Is Magnesium A Metal Metalloid Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.