Is Magnesium A Metal Nonmetal Or Metalloid
Kalali
Mar 12, 2025 · 5 min read
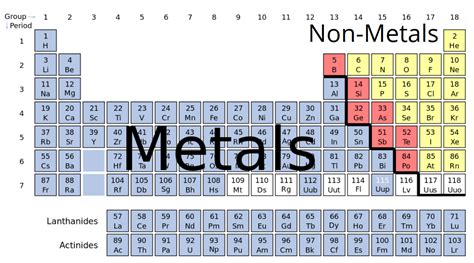
Table of Contents
Is Magnesium a Metal, Nonmetal, or Metalloid? A Comprehensive Look
Magnesium, a ubiquitous element found in numerous biological processes and industrial applications, often sparks curiosity regarding its classification within the periodic table. Is it a metal, a nonmetal, or a metalloid? The answer, unequivocally, is metal. However, a deeper dive reveals the nuances that distinguish magnesium from other metals and highlight its unique properties. This comprehensive article will explore the characteristics of magnesium, definitively classifying it as a metal while examining the properties that define its metallic nature and its interesting exceptions.
Understanding the Classification System
Before we delve into the specifics of magnesium, it's crucial to understand the fundamental differences between metals, nonmetals, and metalloids. These classifications are based on elements' properties, particularly their:
- Electrical Conductivity: Metals are excellent conductors of electricity, while nonmetals are poor conductors (or insulators). Metalloids exhibit intermediate conductivity.
- Thermal Conductivity: Similar to electrical conductivity, metals efficiently conduct heat, whereas nonmetals are poor conductors. Metalloids again show intermediate behavior.
- Malleability and Ductility: Metals are malleable (can be hammered into sheets) and ductile (can be drawn into wires), whereas nonmetals are typically brittle. Metalloids exhibit a mix of properties.
- Luster: Metals generally possess a characteristic metallic luster or shine, while nonmetals lack this luster. Metalloids can sometimes display a metallic luster.
- Reactivity: Metals tend to readily lose electrons in chemical reactions, while nonmetals readily gain electrons. Metalloids exhibit varying reactivity.
Magnesium: A Definitive Metal
Magnesium (Mg), atomic number 12, sits squarely in the alkaline earth metal group (Group 2) of the periodic table. This placement alone strongly indicates its metallic nature. Let's examine its properties in the context of the classification criteria outlined above:
1. Excellent Electrical and Thermal Conductivity
Magnesium is a very good conductor of both electricity and heat. Its free-moving valence electrons facilitate the easy flow of charge and energy, a hallmark characteristic of metals. This conductivity is exploited in numerous applications, including lightweight alloys for automotive parts and electronics.
2. Malleability and Ductility
While not as malleable or ductile as some other metals like gold or copper, magnesium is still considered malleable and ductile. It can be worked and shaped, although it requires careful handling due to its reactivity. This property allows for its use in various manufacturing processes.
3. Metallic Luster
Magnesium possesses a distinct silvery-white metallic luster. This shine is a direct result of the interaction of light with its electron sea, a typical feature observed in metals. However, it's important to note that exposure to air can lead to the formation of a dull oxide layer, obscuring this luster.
4. Chemical Reactivity: A Notable Exception
Here, magnesium demonstrates a property that might seem at odds with its classification as a metal. Magnesium is relatively reactive, particularly with oxygen and acids. This reactivity leads to the formation of magnesium oxide (MgO) and magnesium salts, respectively. This reactivity is a consequence of its low electronegativity and its tendency to readily lose two electrons to achieve a stable electron configuration. While this reactivity might seem unusual, it's important to remember that reactivity is a spectrum and many metals exhibit varying degrees of it. For instance, alkali metals are significantly more reactive than magnesium.
5. Other Metallic Properties
Magnesium exhibits several other properties consistent with its metallic classification:
- High melting and boiling points: Reflecting strong metallic bonding.
- Crystalline structure: Magnesium adopts a hexagonal close-packed crystal structure, typical of many metals.
- Formation of alloys: Magnesium readily forms alloys with other metals, enhancing its strength and other properties. This ability to form alloys is a crucial aspect of its industrial use.
Addressing Potential Misconceptions
Some might be tempted to misclassify magnesium due to its reactivity. However, reactivity is a property that varies significantly across metals and doesn't negate an element's fundamental metallic nature. The key distinguishing factors remain electrical and thermal conductivity, malleability, ductility, and metallic luster, all of which magnesium exhibits strongly.
Applications of Magnesium: A Testament to its Metallic Properties
The wide range of magnesium applications underscores its valuable metallic properties. These include:
-
Lightweight Alloys: Magnesium's low density makes it ideal for creating lightweight alloys used in the automotive, aerospace, and consumer electronics industries. These alloys offer superior strength-to-weight ratios, crucial for fuel efficiency and performance.
-
Structural Components: In construction, magnesium alloys are used in various structural components due to their strength and light weight.
-
Biomedical Applications: Magnesium's biocompatibility makes it suitable for various biomedical applications, such as biodegradable implants and drug delivery systems.
-
Electronics: Magnesium's electrical conductivity makes it a valuable component in electronic devices, including batteries and circuit boards.
-
Pyrotechnics: Magnesium's brilliant white flame when burned is used in fireworks and flares.
-
Photography: Magnesium was historically used in flash photography due to its bright light when ignited.
Conclusion: Magnesium – Undeniably a Metal
In conclusion, the evidence overwhelmingly supports the classification of magnesium as a metal. While its reactivity might seem to deviate from the typical perception of a metal, the defining characteristics of electrical and thermal conductivity, malleability, ductility, and metallic luster unequivocally place magnesium within the metallic category. Its diverse applications, from lightweight alloys to biomedical devices, further highlight its crucial role in various sectors, proving its metallic nature and utility. The nuanced properties of magnesium, including its relatively high reactivity compared to some other metals, simply add to the rich tapestry of its unique characteristics within the broader family of metallic elements. Therefore, any doubts surrounding its classification should be readily dispelled by its comprehensive alignment with all the defining features of a metallic element.
Latest Posts
Latest Posts
-
How Many Packages Of 0 06 Equals 3 Oz
Jul 10, 2025
-
Red With Red Violet And Red Orange Color Combination
Jul 10, 2025
-
2 3 E 4 5 I 6 8
Jul 10, 2025
-
How Do You Write 100 As A Decimal
Jul 10, 2025
-
The Answer To Multiplication Problem Is Called What
Jul 10, 2025
Related Post
Thank you for visiting our website which covers about Is Magnesium A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.