Is Magnesium A Metal Or Nonmetal Or Metalloid
Kalali
Mar 27, 2025 · 5 min read
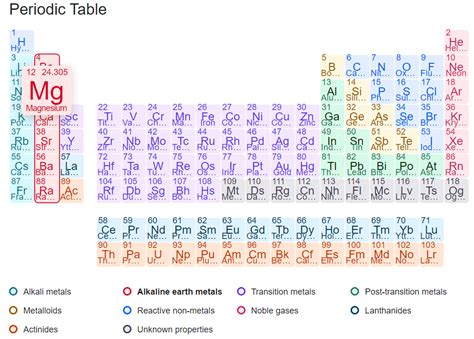
Table of Contents
Is Magnesium a Metal, Nonmetal, or Metalloid? A Deep Dive into its Properties
Magnesium, a silvery-white element found abundantly in the Earth's crust, often sparks curiosity about its classification. Is it a metal, nonmetal, or metalloid? The answer is clear: magnesium is a metal. But understanding why it's classified as a metal requires a deeper exploration of its physical and chemical properties. This article will delve into the characteristics that firmly place magnesium within the metal category, exploring its atomic structure, reactivity, and various applications.
Understanding the Periodic Table and Element Classification
Before diving into magnesium's properties, let's briefly review how elements are classified. The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Elements are broadly categorized into metals, nonmetals, and metalloids (also known as semimetals).
-
Metals: Typically found on the left and center of the periodic table, metals are characterized by their excellent electrical and thermal conductivity, malleability (ability to be hammered into sheets), ductility (ability to be drawn into wires), and metallic luster (shiny appearance). They readily lose electrons to form positive ions (cations).
-
Nonmetals: Located on the right side of the periodic table, nonmetals generally exhibit poor electrical and thermal conductivity, are brittle, and lack metallic luster. They tend to gain electrons to form negative ions (anions).
-
Metalloids: Situated between metals and nonmetals, metalloids possess properties of both. Their conductivity can vary depending on conditions, and they show a mixture of metallic and nonmetallic characteristics.
Magnesium's Defining Metallic Properties
Magnesium's position in the periodic table, Group 2 (alkaline earth metals), immediately suggests its metallic nature. Let's examine the key properties that solidify its classification as a metal:
1. Excellent Electrical and Thermal Conductivity
Magnesium is a remarkably good conductor of both electricity and heat. This property stems from the loosely held valence electrons in its atomic structure. These electrons are free to move throughout the metal lattice, facilitating the flow of both electrical current and thermal energy. This high conductivity is extensively utilized in various applications, as we'll see later.
2. Malleability and Ductility
Magnesium exhibits significant malleability and ductility. It can be readily hammered into thin sheets (malleability) and drawn into wires (ductility) without fracturing. This is a direct consequence of the metallic bonding present in its crystal structure, allowing the metal atoms to slide past each other relatively easily under stress.
3. Metallic Luster
Magnesium possesses a characteristic silvery-white metallic luster. This shiny appearance is a result of the interaction of light with the free electrons in its metallic lattice. The electrons absorb and re-emit light, giving magnesium its distinctive metallic sheen. This luster is often used as an indicator of a material's metallic character.
4. Electropositivity and Ionization
Magnesium is highly electropositive, meaning it readily loses electrons to achieve a stable electron configuration. Its low ionization energy (the energy required to remove an electron) allows it to easily form Mg²⁺ ions, exhibiting typical metallic behavior. This tendency to lose electrons and form positive ions is a defining characteristic of metals.
5. Reaction with Acids and Other Reagents
Magnesium's reactivity with acids is a clear demonstration of its metallic character. It readily reacts with dilute acids, such as hydrochloric acid (HCl), to produce magnesium salts and hydrogen gas. This reaction is a classic example of a metal displacing hydrogen from an acid.
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
Furthermore, magnesium reacts with other reagents, such as oxygen, to form oxides. This reactivity, while demonstrating its chemical activity, doesn't negate its metallic nature; rather, it highlights its tendency to lose electrons and form ionic compounds.
Debunking Misconceptions: Why Magnesium Isn't a Metalloid or Nonmetal
Given the broad range of properties observed in some elements, it's essential to address any potential misconceptions about magnesium's classification:
-
Not a Metalloid: Magnesium lacks the variable conductivity and semiconducting properties typically associated with metalloids. Its high conductivity at room temperature and the consistent behavior of its electrons clearly distinguish it from metalloids.
-
Not a Nonmetal: Magnesium starkly contrasts with nonmetals in its physical and chemical properties. Unlike nonmetals, it is a good conductor of electricity and heat, it's malleable and ductile, and it readily loses electrons to form positive ions. These properties definitively exclude magnesium from the nonmetal category.
Applications Leveraging Magnesium's Metallic Properties
The unique combination of lightweight, strength, and reactivity makes magnesium invaluable in various applications:
1. Alloying Agent:
Magnesium's low density and ability to form strong alloys with other metals (e.g., aluminum) makes it crucial in aerospace and automotive industries. Magnesium alloys are used in aircraft components, car parts, and other applications requiring lightweight, high-strength materials.
2. Structural Materials:
Magnesium's strength-to-weight ratio makes it suitable for constructing lightweight structural components in various industries. Its use in ladders, scaffolding, and other applications where weight reduction is critical showcases its versatility.
3. Sacrificial Anodes:
Magnesium's high reactivity is exploited in corrosion protection. Magnesium anodes are used in cathodic protection systems to protect other metals from corrosion. The magnesium acts as a sacrificial anode, corroding preferentially to protect the more valuable metal.
4. Pyrotechnics:
Magnesium's brilliant white light upon combustion makes it a valuable component in fireworks and flares. This characteristic is used to generate intense, bright light in pyrotechnic displays.
5. Chemical Industry:
Magnesium is a vital reducing agent in the chemical industry. It's used in the production of various metals and other chemicals, where its ability to readily lose electrons is exploited for chemical transformations.
Conclusion: Magnesium – A Definitive Metal
The evidence overwhelmingly supports the classification of magnesium as a metal. Its excellent electrical and thermal conductivity, malleability, ductility, metallic luster, electropositivity, and reactions with acids and other reagents align perfectly with the defining characteristics of metals. Any arguments suggesting otherwise are easily refuted by its fundamental properties and widespread applications. Its unique combination of properties makes magnesium a versatile and essential element in a wide range of industries, highlighting its importance in modern technology and beyond. The next time you encounter magnesium, remember its true metallic identity and the multitude of applications this remarkable element supports.
Latest Posts
Latest Posts
-
1 In 25 As A Percentage
Mar 30, 2025
-
For Each Action There Is A Reaction
Mar 30, 2025
-
Why Do Flowers Contain More Stamen Than Pistils
Mar 30, 2025
-
Why Is The Entropy Change Negative For Ring Closures
Mar 30, 2025
-
How Tall Is 69 5 Inches In Feet
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Magnesium A Metal Or Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
