Is Nitric Acid A Strong Electrolyte
Kalali
Apr 01, 2025 · 5 min read
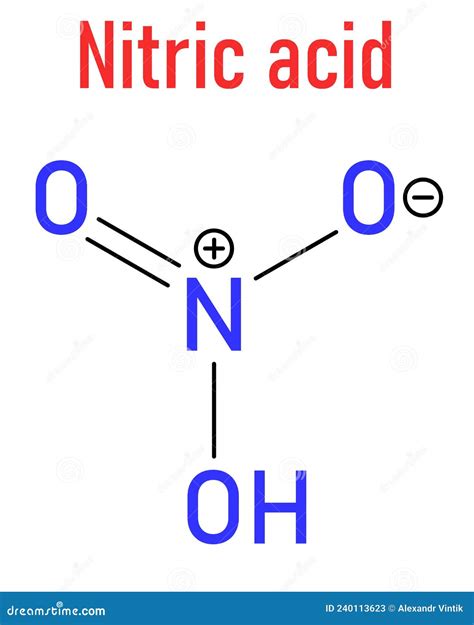
Table of Contents
Is Nitric Acid a Strong Electrolyte? A Deep Dive into Acid Strength and Conductivity
Nitric acid (HNO₃), a highly corrosive and toxic strong acid, is a staple in various chemical processes. Understanding its properties, particularly its electrolytic strength, is crucial for anyone working with this substance. This comprehensive article delves into the question: Is nitric acid a strong electrolyte? We will explore the concept of strong electrolytes, delve into the dissociation of nitric acid, examine its conductivity, and discuss its applications based on its electrolytic nature.
Understanding Strong Electrolytes
Before we definitively answer the question regarding nitric acid, let's establish a clear understanding of what constitutes a strong electrolyte. A strong electrolyte is a substance that completely, or almost completely, dissociates into its constituent ions when dissolved in a solvent, typically water. This dissociation generates a large number of freely moving ions, which are responsible for the solution's ability to conduct electricity. The higher the degree of dissociation, the stronger the electrolyte and the better its conductivity.
Key characteristics of strong electrolytes:
- High degree of ionization: Essentially all molecules dissociate into ions.
- High electrical conductivity: Solutions of strong electrolytes exhibit high conductivity due to the abundance of charge carriers.
- Complete dissociation: The equilibrium of the dissociation reaction heavily favors the ionic products.
Conversely, weak electrolytes only partially dissociate in solution, leading to a lower concentration of ions and, therefore, lower conductivity.
The Dissociation of Nitric Acid
Nitric acid, in aqueous solution, undergoes complete dissociation, releasing a proton (H⁺) and a nitrate ion (NO₃⁻):
HNO₃(aq) → H⁺(aq) + NO₃⁻(aq)
This complete dissociation is the hallmark of a strong acid. The equilibrium lies far to the right, meaning that almost all the nitric acid molecules present dissociate into ions. This is in stark contrast to weak acids, such as acetic acid (CH₃COOH), which only partially dissociate.
The complete dissociation is due to the relatively weak N-O bond in the nitric acid molecule and the high stability of the nitrate ion (NO₃⁻). The nitrate ion is resonance-stabilized, meaning its charge is delocalized across multiple oxygen atoms, resulting in a lower energy state and increased stability. This high stability of the products drives the dissociation reaction towards completion.
Factors Affecting Dissociation
While the dissociation of nitric acid is essentially complete in dilute solutions, some factors can slightly influence the extent of ionization:
- Concentration: At extremely high concentrations, the interionic forces between the ions can slightly hinder complete dissociation. However, even at high concentrations, nitric acid remains a strong electrolyte.
- Temperature: Temperature affects the dissociation equilibrium. Increased temperature generally enhances the dissociation process, although the effect is relatively minor for nitric acid since it's already highly dissociated.
- Solvent: The solvent's polarity and dielectric constant play a role. Water, being a highly polar solvent, effectively stabilizes the ions produced during dissociation.
Conductivity as Evidence of Strong Electrolyte Nature
The high electrical conductivity of nitric acid solutions provides compelling evidence of its strong electrolyte nature. The abundance of freely moving H⁺ and NO₃⁻ ions allows the solution to readily conduct an electric current. This conductivity can be easily demonstrated using a simple conductivity apparatus. A solution of nitric acid will show significantly higher conductivity compared to a solution of a weak acid at the same concentration.
The conductivity measurements directly reflect the concentration of ions in the solution. Since nitric acid completely dissociates, its conductivity is directly proportional to its concentration. This linear relationship between conductivity and concentration further supports its classification as a strong electrolyte.
Applications Leveraging Nitric Acid's Electrolytic Properties
The strong electrolytic nature of nitric acid is crucial in numerous applications. Its ability to conduct electricity and readily provide ions is exploited in various industrial processes and scientific experiments:
- Electroplating: Nitric acid is used in some electroplating processes, though often in combination with other electrolytes. The conductivity provided by nitric acid ensures a smooth and efficient deposition of metals onto surfaces.
- Chemical Synthesis: Nitric acid's high reactivity due to its strong dissociation is essential in numerous chemical syntheses. It acts as a strong oxidizing agent and proton source in various organic and inorganic reactions.
- Etching and Cleaning: The corrosive nature of nitric acid, stemming from its strong electrolytic properties, is used in etching metals and cleaning various materials. This application benefits from the high concentration of reactive ions in solution.
- Explosives Manufacturing: Nitric acid is a key component in the synthesis of various explosives, notably nitrocellulose and nitroglycerin. The strong oxidizing power and ease of ion generation are critical for these reactions.
- Analytical Chemistry: Its strong electrolyte nature makes nitric acid useful in various analytical techniques, such as titrations and electrochemical methods. The high conductivity enables accurate and reliable measurements.
Comparing Nitric Acid to Other Electrolytes
To further solidify the understanding of nitric acid's strength as an electrolyte, let's compare it with other substances:
- Strong Acids: Other strong acids, such as hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), also exhibit strong electrolytic behavior, exhibiting near-complete dissociation in aqueous solutions. Their conductivity is comparable to that of nitric acid.
- Weak Acids: Weak acids, such as acetic acid (CH₃COOH) and carbonic acid (H₂CO₃), demonstrate significantly lower conductivity compared to nitric acid due to their partial dissociation.
- Salts: Many salts, such as sodium chloride (NaCl) and potassium nitrate (KNO₃), are strong electrolytes. Their complete dissociation into ions results in high conductivity.
- Nonelectrolytes: Nonelectrolytes, such as sugar (sucrose) and ethanol, do not dissociate into ions in solution and hence exhibit negligible conductivity.
Conclusion: Nitric Acid is Indeed a Strong Electrolyte
In conclusion, the evidence overwhelmingly supports the assertion that nitric acid is a strong electrolyte. Its complete dissociation in aqueous solutions, high electrical conductivity, and numerous applications leveraging its electrolytic properties all point to this definitive classification. The high stability of the nitrate ion and the strong tendency for proton release drive the dissociation process towards completion, resulting in a solution rich in charge carriers and capable of conducting electricity efficiently. Understanding this fundamental property of nitric acid is crucial for safe and effective handling, as well as its application in various industrial and scientific contexts. Further research and practical experimentation continually reinforce this classification. The scientific community widely accepts nitric acid as a prime example of a strong electrolyte.
Latest Posts
Latest Posts
-
50 30 Written As A Product Of Two Factors
Apr 02, 2025
-
What Percentage Is 11 Out Of 12
Apr 02, 2025
-
How Many Fahrenheit Is 1 Degree Celsius
Apr 02, 2025
-
What Percent Of 6 Is 4
Apr 02, 2025
-
Lowest Common Multiple Of 5 And 10
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Nitric Acid A Strong Electrolyte . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
