Is Oil And Water A Homogeneous Mixture
Kalali
Mar 17, 2025 · 5 min read
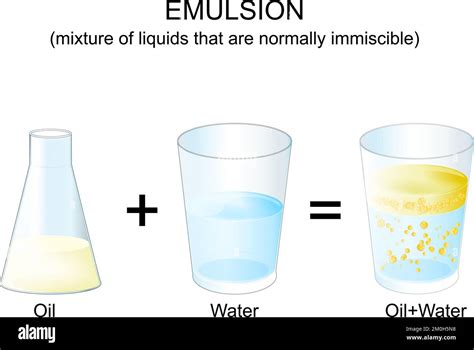
Table of Contents
Is Oil and Water a Homogeneous Mixture? A Deep Dive into Mixture Types
The simple answer is no, oil and water are not a homogeneous mixture. Understanding why requires a delve into the fundamental properties of matter and the definitions of different mixture types. This article will explore the concept of homogeneous and heterogeneous mixtures, examining the distinct characteristics of oil and water to definitively answer this question. We'll also explore the scientific principles behind their immiscibility and the implications of this property in various contexts.
Understanding Mixtures: Homogeneous vs. Heterogeneous
Before we dissect the oil-and-water conundrum, let's clarify the definitions of homogeneous and heterogeneous mixtures. A mixture is a substance comprising two or more components not chemically bonded. The crucial difference lies in the uniformity of these components:
-
Homogeneous Mixture: A homogeneous mixture has a uniform composition throughout. This means that at a macroscopic level (visible to the naked eye), the components are indistinguishable. Examples include saltwater, air (a mixture of gases), and sugar dissolved in water. No matter which part of the sample you take, the composition remains consistent.
-
Heterogeneous Mixture: A heterogeneous mixture has a non-uniform composition. The different components are visible and easily distinguishable. Examples include sand and water, oil and water (as we'll explore), and a salad. The composition varies depending on the sample location.
The Immiscibility of Oil and Water: A Tale of Polarity
The reason oil and water don't mix boils down to the concept of polarity. Water (H₂O) is a polar molecule. This means it has a slightly positive end and a slightly negative end due to the uneven distribution of electrons within the molecule. This polarity allows water molecules to form strong hydrogen bonds with each other, creating a cohesive structure.
Oil, on the other hand, is typically composed of nonpolar molecules. These molecules have an even distribution of electrons, resulting in no significant positive or negative poles. Nonpolar molecules don't interact strongly with polar molecules like water.
This difference in polarity is the primary reason for the immiscibility of oil and water. The strong attraction between water molecules prevents the oil molecules from intermingling. The oil molecules are repelled by the water molecules, leading to the formation of distinct layers. This is a classic example of the principle "like dissolves like." Polar substances dissolve in polar solvents, while nonpolar substances dissolve in nonpolar solvents.
Exploring the Intermolecular Forces: A Deeper Look
The behavior of oil and water is governed by various intermolecular forces. Water molecules exhibit strong hydrogen bonding, while oil molecules are primarily held together by van der Waals forces, which are significantly weaker. The stronger hydrogen bonds in water dominate the interaction, preventing the weaker van der Waals forces of the oil molecules from effectively integrating into the water structure. This fundamental difference in intermolecular forces reinforces their immiscibility.
Visual Evidence: The Distinct Layers
The visual evidence of oil and water's heterogeneity is undeniable. When you combine them in a container, two distinct layers are immediately apparent. The less dense oil floats on top of the denser water. This observation further reinforces the conclusion that they do not form a homogeneous mixture. The lack of uniform distribution across the entire volume is a defining characteristic of a heterogeneous mixture.
Beyond Visual Observation: Microscopic Examination
While visually apparent, we can delve deeper into the heterogeneous nature of oil and water using microscopic techniques. Microscopy would reveal the distinct boundaries between the oil and water phases, with no molecular-level intermixing. This is in stark contrast to a homogeneous mixture where even microscopic examination would show a uniform distribution of components.
Applications and Implications of Immiscibility
The immiscibility of oil and water has significant implications across various fields:
-
Environmental Science: Oil spills are a major environmental concern. The immiscibility of oil and water makes cleaning up oil spills challenging, as the oil doesn't readily disperse in water. This necessitates specialized techniques and technologies for remediation.
-
Food Science: Emulsifiers are crucial in food science to create stable mixtures of oil and water, such as in mayonnaise or salad dressings. These emulsifiers act as bridges, reducing the interfacial tension between oil and water and allowing for a more uniform dispersion.
-
Chemistry and Chemical Engineering: Understanding the principles of polarity and immiscibility is crucial in various chemical processes, including separations, extractions, and the design of chemical reactors. The separation of oil and water is a common process in many industrial settings.
-
Biology: The cell membrane, a critical component of all living cells, is a complex structure that maintains a separation between the aqueous interior of the cell and the external aqueous environment. This separation is essential for maintaining cellular function. The principles underlying the immiscibility of oil and water play a role in the stability and function of cell membranes.
Addressing Common Misconceptions
It's important to address some common misconceptions related to oil and water mixtures:
-
Stirring Doesn't Create Homogeneity: Vigorous stirring may create an emulsion – a temporary suspension of tiny oil droplets in water. However, this is still a heterogeneous mixture because the oil and water haven't dissolved into each other at a molecular level. Given enough time, the mixture will separate again.
-
Emulsifiers Alter, but Don't Eliminate, Heterogeneity: As mentioned earlier, emulsifiers can create stable emulsions. However, even in an emulsion, the oil and water remain distinct phases; they are merely finely dispersed. The fundamental heterogeneity remains.
Conclusion: A Definitive Answer
In conclusion, oil and water are unequivocally a heterogeneous mixture. Their distinct physical properties, primarily their contrasting polarities and intermolecular forces, prevent them from forming a homogenous solution. The visual observation of separate layers, microscopic confirmation of distinct phases, and the necessity of emulsifiers to achieve even a temporary suspension all point towards the heterogeneous nature of this combination. Understanding this principle is fundamental across diverse scientific disciplines and has significant practical applications in various fields. The immiscibility of oil and water is not merely a simple observation; it's a testament to the profound influence of molecular interactions on macroscopic properties.
Latest Posts
Latest Posts
-
What Number Is 15 Of 80
Mar 17, 2025
-
136 Inches Is How Many Feet
Mar 17, 2025
-
Negative Fraction Divided By Negative Fraction
Mar 17, 2025
-
Equations For Photosynthesis And Cellular Respiration
Mar 17, 2025
-
Which Is Greater 0 25 Or 0 5
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Oil And Water A Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
