Is Pure Water A Homogeneous Mixture
Kalali
Mar 17, 2025 · 4 min read
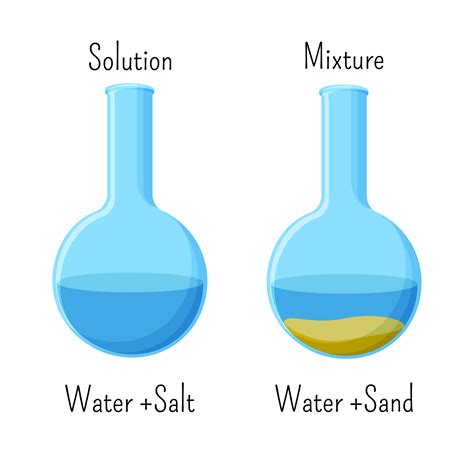
Table of Contents
- Is Pure Water A Homogeneous Mixture
- Table of Contents
- Is Pure Water a Homogeneous Mixture? A Deep Dive into the Nature of Water
- Understanding the Terminology: Pure Substances vs. Mixtures
- The Chemical Composition of Pure Water
- The Physical Properties of Pure Water: A Homogeneous Appearance
- Microscopic View: Molecular Arrangement in Pure Water
- Distinguishing Pure Water from Impure Water (Solutions)
- Addressing Potential Counterarguments
- Conclusion: Pure Water is NOT a Homogeneous Mixture, it is a Pure Substance
- Latest Posts
- Latest Posts
- Related Post
Is Pure Water a Homogeneous Mixture? A Deep Dive into the Nature of Water
The question, "Is pure water a homogeneous mixture?" might seem deceptively simple. After all, water looks uniform to the naked eye. However, delving into the chemistry and physics of water reveals a more nuanced answer. Understanding this requires exploring the definitions of pure substances, mixtures, and the unique properties of water at the molecular level. This comprehensive article will dissect the concept, exploring various aspects to definitively answer the question and provide a thorough understanding of water's nature.
Understanding the Terminology: Pure Substances vs. Mixtures
Before tackling the core question, let's clearly define our terms. A pure substance is a form of matter that has a constant composition and properties throughout a given sample. It cannot be separated into component parts by physical methods. Examples include elements (like oxygen or gold) and compounds (like water or sodium chloride).
A mixture, conversely, comprises two or more substances that are physically combined but not chemically bonded. Mixtures can be either homogeneous or heterogeneous. A homogeneous mixture has a uniform composition throughout; its individual components are not visually distinguishable. Think of saltwater: the salt is dissolved evenly throughout the water. A heterogeneous mixture, on the other hand, has a non-uniform composition, with visibly distinct components. A salad is a classic example of a heterogeneous mixture.
The Chemical Composition of Pure Water
Pure water, chemically represented as H₂O, is a compound, not a mixture. It's formed by the chemical bonding of two hydrogen atoms and one oxygen atom through covalent bonds. These bonds are strong electrostatic forces that hold the atoms together in a fixed ratio. This fixed ratio defines the constant composition of pure water. You will always find two hydrogen atoms for every oxygen atom in a pure water molecule, regardless of the sample size. This consistent ratio is a hallmark of pure substances, differentiating them from mixtures.
The Physical Properties of Pure Water: A Homogeneous Appearance
At the macroscopic level, pure water appears uniform. Its physical properties, such as density, boiling point, and freezing point, remain consistent throughout the sample. This uniformity further supports the idea that pure water is not a mixture. If it were a mixture, we would expect variations in these properties depending on the concentration of its components. The observation of consistent properties throughout is a clear indicator of homogeneity.
Microscopic View: Molecular Arrangement in Pure Water
At the microscopic level, pure water consists of water molecules (H₂O) interacting through hydrogen bonds. Hydrogen bonds are relatively weak intermolecular forces compared to the covalent bonds within the water molecule. These bonds create a dynamic network of interacting water molecules, with molecules constantly moving and forming and breaking hydrogen bonds. This dynamic nature does not affect the overall homogeneity of the sample. The consistent ratio of hydrogen to oxygen atoms remains constant, making it chemically pure and physically homogeneous.
Distinguishing Pure Water from Impure Water (Solutions)
It's crucial to differentiate pure water from water containing dissolved substances. Tap water, for example, is not pure water. It contains dissolved minerals, gases, and possibly even pollutants. This makes tap water a homogeneous mixture, not a pure substance. The dissolved substances are distributed evenly throughout the water, giving it a uniform appearance, but its composition is not constant.
Seawater presents a similar case. The high concentration of salts and other dissolved minerals alters the physical properties of the water, making it distinctly different from pure water. This mixture, while homogeneous, is not the same as pure water. The key distinction lies in the presence of other substances affecting the constant composition defining a pure substance.
Addressing Potential Counterarguments
Some might argue that the presence of isotopes of hydrogen and oxygen (deuterium, tritium, and oxygen-18) in naturally occurring water makes it a mixture. While this is technically true, the isotopic variations are present in trace amounts and do not significantly alter the overall chemical and physical properties of water. Considering this small isotopic variation, it does not affect the classification of pure water as a homogeneous substance. The isotopic composition variations are negligible in the context of defining a pure substance versus a mixture.
Conclusion: Pure Water is NOT a Homogeneous Mixture, it is a Pure Substance
Based on the rigorous examination of its chemical composition, physical properties, and microscopic structure, we can definitively conclude that pure water is not a homogeneous mixture. It is a pure substance, a compound formed from the chemical bonding of hydrogen and oxygen atoms in a fixed ratio. While water in the natural world often contains dissolved substances, transforming it into a homogeneous mixture, pure water itself maintains a constant composition and exhibits consistent properties throughout a sample, meeting the criteria of a pure substance. The apparent homogeneity at the macroscopic level arises from the consistent composition at the molecular level, not from a mixture of substances. Therefore, the answer to the central question is a clear and definitive "no".
Latest Posts
Latest Posts
-
How Much Is 1 1 4 Cup Of Water
Mar 17, 2025
-
How Tall Is 76 Inches In Feet
Mar 17, 2025
-
8 Pounds Is How Many Ounces
Mar 17, 2025
-
How Many Cups Is 12 Fluid Ounces
Mar 17, 2025
-
32 Fl Oz Is How Many Cups
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Pure Water A Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
