Is Salt Water A Pure Substance
Kalali
Mar 10, 2025 · 5 min read
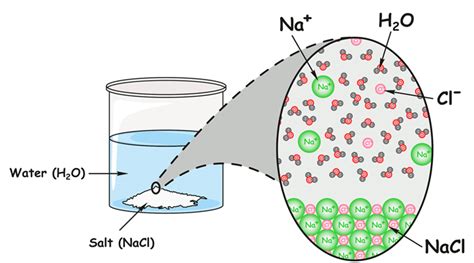
Table of Contents
Is Salt Water a Pure Substance? A Deep Dive into Mixtures and Solutions
The question, "Is saltwater a pure substance?" seems simple at first glance. However, understanding the answer requires delving into the fundamental concepts of chemistry, specifically differentiating between pure substances and mixtures. This article will explore the composition of saltwater, examining its properties and explaining why it's definitively classified as a mixture, not a pure substance. We'll also touch upon various types of mixtures and the crucial distinctions between them.
Understanding Pure Substances
Before we tackle saltwater, let's define a pure substance. A pure substance is a form of matter that has a constant composition (a fixed ratio of elements) and properties throughout the sample. It cannot be separated into other kinds of matter by any physical process. Pure substances are further categorized into:
Elements:
Elements are the fundamental building blocks of matter. They are substances that cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), hydrogen (H), and iron (Fe). Each element is defined by its atomic number, representing the number of protons in its nucleus.
Compounds:
Compounds are pure substances formed when two or more elements chemically combine in a fixed ratio. This combination results in a new substance with properties distinct from its constituent elements. For example, water (H₂O) is a compound formed from the combination of hydrogen and oxygen. The properties of water are vastly different from those of hydrogen and oxygen gases. The ratio of hydrogen to oxygen in water is always 2:1. This fixed ratio is a defining characteristic of compounds. NaCl (sodium chloride) is another example, where the ratio of Sodium to Chlorine is always 1:1.
Understanding Mixtures
Unlike pure substances, mixtures are combinations of two or more substances that are physically combined but not chemically bonded. The components of a mixture retain their individual properties, and their proportions can vary. Mixtures can be separated into their constituent parts by physical methods, such as filtration, distillation, or evaporation. There are two main types of mixtures:
Homogeneous Mixtures:
In a homogeneous mixture, the components are uniformly distributed throughout the mixture. The mixture appears to be a single phase, meaning it has a uniform composition and properties throughout. Saltwater is an example of a homogeneous mixture. At the macroscopic level, the salt and water appear completely blended. However, at the microscopic level, we can still identify distinct water molecules and sodium and chloride ions. Other examples include air (a mixture of gases) and sugar dissolved in water.
Heterogeneous Mixtures:
In a heterogeneous mixture, the components are not uniformly distributed. Different parts of the mixture have different compositions and properties. Examples include sand and water, oil and water, and a salad. You can easily visually distinguish the different components.
Why Saltwater is a Mixture
Now, let's return to the central question: Is saltwater a pure substance? The answer is a resounding no. Saltwater is a homogeneous mixture. Here's why:
-
Variable Composition: The ratio of salt (sodium chloride, NaCl) to water (H₂O) in saltwater can vary widely depending on the source. Ocean water, for example, has a different salt concentration than water from a salt lake or a salt solution prepared in a lab. This variability in composition is a key characteristic of mixtures, unlike the fixed composition of pure substances.
-
Physical Combination: Salt and water are combined physically. When salt is added to water, it dissolves, forming ions (Na⁺ and Cl⁻) that are surrounded by water molecules. However, no new chemical bonds are formed between the salt and water molecules. This is a crucial distinction between mixtures and compounds. The chemical identities of both salt and water are preserved.
-
Separation by Physical Means: The components of saltwater can be separated by physical means. For example, evaporation will leave behind solid salt crystals, demonstrating that the salt and water were never chemically bound. Distillation, another physical separation technique, would also recover both the pure water and salt separately.
Microscopic View of Saltwater
At a microscopic level, the homogeneous nature of saltwater becomes clearer. The salt crystals dissolve in water, breaking down into positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻). These ions are surrounded by water molecules, a process called hydration. The water molecules interact with the ions through electrostatic forces, keeping them dispersed evenly throughout the solution. Despite this interaction, the individual water molecules and the sodium and chloride ions retain their chemical identity. No new chemical species are formed.
Further Distinguishing Features of Mixtures
To further solidify the understanding of why saltwater isn't a pure substance, let's highlight additional distinguishing features of mixtures:
-
Retention of Individual Properties: In saltwater, both water and salt retain their individual properties. Water remains a liquid, and salt retains its salty taste. This contrasts with compounds where the properties of the constituent elements are often dramatically altered.
-
No Fixed Melting/Boiling Point: Pure substances have fixed melting and boiling points. Saltwater, however, has a boiling point higher than that of pure water and a freezing point lower than that of pure water. These changes in physical properties are dependent on the concentration of salt in the water.
-
Easy Separation: As previously mentioned, the components of saltwater can be easily separated through simple physical processes, emphasizing the absence of a chemical bond between the salt and water.
Conclusion: Saltwater as a Homogeneous Mixture
In conclusion, saltwater is unequivocally a homogeneous mixture, not a pure substance. Its variable composition, the physical nature of its combination, the retention of individual properties, and the ease of separation through physical means all point towards this classification. Understanding this distinction is critical for grasping fundamental concepts in chemistry and appreciating the diverse forms matter can take. The seemingly simple question of whether saltwater is a pure substance unveils a rich understanding of mixtures and their behavior, offering a valuable lesson in the world of chemistry. By understanding these fundamental concepts, we can better appreciate the complexity and beauty of the natural world.
Latest Posts
Latest Posts
-
Folder Is To Document As Envelope Is To
Jun 30, 2025
-
How Many Times Does 6 Go Into 100
Jun 30, 2025
-
What Is 453 605 Rounded To The Nearest Thousand
Jun 30, 2025
-
How Many Grams Is A Kilo Of Coke
Jun 30, 2025
-
How Many Tablespoons In A Packet Of Hidden Valley Ranch
Jun 30, 2025
Related Post
Thank you for visiting our website which covers about Is Salt Water A Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.