Is Snow A Solid Liquid Or Gas
Kalali
Mar 31, 2025 · 5 min read
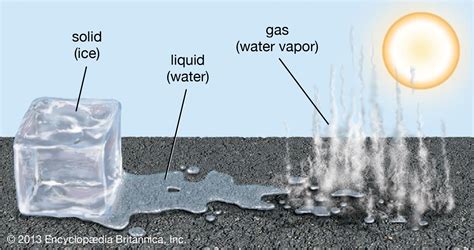
Table of Contents
Is Snow a Solid, Liquid, or Gas? Understanding the Phases of Water
Snow, that beautiful, delicate crystalline wonder blanketing the landscape, often evokes feelings of winter magic and childhood joy. But have you ever stopped to consider its fundamental nature? Is snow a solid, a liquid, or a gas? The answer, as with many scientific questions, isn't quite as straightforward as it might initially seem. Understanding the state of snow requires delving into the fascinating world of water's phases and the processes that govern its transformation.
The Three Main States of Matter: Solid, Liquid, and Gas
Before we classify snow, let's review the basic states of matter. Most substances exist in one of three primary states:
-
Solid: In a solid state, molecules are tightly packed together in a fixed, ordered arrangement. This rigid structure gives solids a definite shape and volume. They resist changes in shape and volume unless significant force is applied. Think of an ice cube – it maintains its shape until it melts.
-
Liquid: In a liquid state, molecules are still close together but have more freedom of movement than in a solid. Liquids have a definite volume but adopt the shape of their container. They flow and can be poured. Water in a glass is a perfect example.
-
Gas: In a gaseous state, molecules are widely dispersed and move rapidly and randomly. Gases have neither a definite shape nor a definite volume; they expand to fill their container. Air is a mixture of gases.
Snow: A Solid Form of Water
The most straightforward answer to the question, "Is snow a solid, liquid, or gas?" is solid. Snow is composed of countless tiny ice crystals, each a beautiful hexagonal structure formed through a process called deposition. Deposition is the direct transition of water vapor (gas) into ice (solid), bypassing the liquid phase. This happens when water vapor in the atmosphere encounters extremely cold temperatures.
The Formation of Snow Crystals: A Microscopic Marvel
The formation of a snowflake begins with a tiny dust particle or ice nucleus in the atmosphere. Water molecules then attach themselves to this nucleus, initially forming a small hexagonal prism. As the crystal falls through the atmosphere, it encounters varying temperatures and humidity levels. These conditions influence the arrangement of water molecules as they attach to the crystal, resulting in the intricate and unique patterns we associate with snowflakes. No two snowflakes are exactly alike!
The Properties of Snow as a Solid
Several characteristics of snow confirm its solid nature:
- Definite shape (though not rigid): While individual snowflakes possess distinct shapes, a snowdrift takes on the shape of its surroundings. However, unlike a liquid, it doesn't readily conform to the shape of any container.
- Definite volume: A given amount of snow occupies a specific volume.
- Resistance to flow: Snow doesn't flow freely like a liquid; it resists deformation until enough pressure is applied.
The Role of Liquid Water in Snow
While snow itself is a solid, it's crucial to acknowledge the role liquid water plays in its properties and behavior. Snow is not completely dry ice; it often contains varying amounts of liquid water held within the spaces between the ice crystals. This liquid water content significantly influences:
-
Density: The density of snow varies greatly depending on the amount of liquid water present. Fresh, powdery snow is less dense than wet, heavy snow.
-
Compaction: The liquid water in snow allows for compaction. Pressure can cause the ice crystals to rearrange and the liquid water to fill the spaces between them, resulting in denser, more compact snow.
-
Melting: When the temperature rises above freezing, the liquid water content increases, eventually leading to the melting of the snow.
The Subtleties of Phase Transitions in Snow
The reality is more nuanced than a simple solid classification. Snow is a complex system constantly interacting with its environment. Phase transitions (changes in state) are constantly occurring at a microscopic level, even within a seemingly solid mass of snow.
-
Sublimation: As the sun shines on snow, some of the ice crystals can directly transition into water vapor (gas) through sublimation, a process that is the opposite of deposition. This is why snow can disappear even when the temperature remains below freezing.
-
Melting and Freezing: Even in freezing temperatures, there is a continuous exchange between ice and liquid water within the snowpack. Small amounts of ice may melt, and liquid water may refreeze, depending on the ambient temperature and other factors.
Snow's Unique Properties: More Than Just Solid Ice
The properties of snow go beyond its classification as a solid. Its intricate crystalline structure, its capacity to hold liquid water, and its susceptibility to phase transitions at the microscopic level all contribute to its uniqueness and influence its behavior in the environment. This behavior impacts many natural processes, including:
-
Water cycle: Snow acts as a crucial reservoir of water, releasing it gradually as it melts. This slow release is essential for sustaining ecosystems.
-
Avalanches: The combination of solid ice crystals, liquid water, and changing temperature conditions makes snow susceptible to avalanches, a significant natural hazard in mountainous regions.
-
Insulation: The air spaces between snow crystals provide significant insulation, protecting plants and animals from extreme cold.
-
Reflection: Snow's high albedo (reflectivity) means it reflects a significant portion of solar radiation back into space, playing a crucial role in Earth's climate.
Conclusion: A Dynamic System
While we can definitively state that snow is primarily a solid, its complexity extends beyond this simple classification. It's a dynamic system, a constantly shifting balance between solid ice, liquid water, and gaseous water vapor. Its properties and behaviors are a captivating demonstration of the intricate interplay of temperature, pressure, and phase transitions in the natural world. Understanding the intricacies of snow, therefore, involves appreciating its dynamic nature, the microscopic processes occurring within it, and its vital role in the Earth’s systems. This deeper understanding moves beyond a simple identification as a solid and allows us to appreciate the beauty and complexity of this remarkable natural phenomenon.
Latest Posts
Latest Posts
-
How Long Does It Take A Fossil To Form
Apr 02, 2025
-
How Many Inches Is In 15 Cm
Apr 02, 2025
-
How Much Water In 2 Quarts
Apr 02, 2025
-
What Is 8 5 Cm In Inches
Apr 02, 2025
-
42 Feet Is How Many Meters
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Snow A Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
