Is Sulfur A Cation Or Anion
Kalali
Mar 13, 2025 · 5 min read
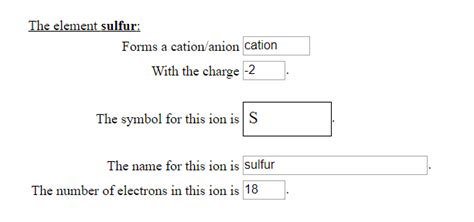
Table of Contents
Is Sulfur a Cation or an Anion? Understanding Sulfur's Ionic Behavior
Sulfur, a nonmetal element found in abundance on Earth, plays a crucial role in various biological and industrial processes. Understanding its chemical behavior, particularly its ionic nature, is fundamental to comprehending its diverse applications. This article delves deep into the question: is sulfur a cation or an anion? We'll explore sulfur's electron configuration, its tendency to gain or lose electrons, and the formation of various sulfur-containing ions, clarifying its role in different chemical contexts.
Sulfur's Electronic Configuration: The Key to Ion Formation
To determine whether sulfur acts as a cation (positively charged ion) or an anion (negatively charged ion), we must examine its electronic structure. Sulfur (S) has an atomic number of 16, meaning it possesses 16 protons and, in its neutral state, 16 electrons. Its electron configuration is 1s²2s²2p⁶3s²3p⁴. This configuration reveals that the outermost shell (valence shell) contains six electrons. Atoms strive for stability, often achieved by attaining a full outer shell (octet rule), which typically contains eight electrons.
The Octet Rule and Sulfur's Behavior
The octet rule dictates that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their valence shell. Considering sulfur's six valence electrons, it's much more energetically favorable for it to gain two electrons to complete its octet than to lose six electrons. Losing six electrons would require a significant amount of energy, making it highly improbable.
Sulfur as an Anion: The Sulfide Ion (S²⁻)
Because of its strong tendency to gain two electrons to achieve a stable octet, sulfur predominantly forms a negatively charged ion, specifically the sulfide ion (S²⁻). This anion is a fundamental component in various inorganic compounds and plays a significant role in numerous chemical reactions. The sulfide ion's negative charge arises from the two extra electrons it acquires.
Formation of Sulfide Ion: A Detailed Look
The formation of the sulfide ion involves sulfur accepting two electrons from another atom, typically a metal. This electron transfer results in an ionic bond, where the electrostatic attraction between the positively charged metal cation and the negatively charged sulfide anion holds the compound together. For example, in the formation of sodium sulfide (Na₂S), two sodium atoms each lose one electron to become Na⁺ cations, while one sulfur atom gains two electrons to form the S²⁻ anion. The resulting compound is held together by the strong electrostatic forces between the oppositely charged ions.
Examples of Sulfur in its Anionic Form
The sulfide ion (S²⁻) is ubiquitous in various chemical contexts. Its presence is crucial in numerous compounds and reactions. Let’s explore some prominent examples:
1. Metal Sulfides: The Building Blocks of Minerals
Many metal sulfides exist naturally as minerals. These minerals often possess unique properties and play crucial roles in various geological processes. Examples include:
- Iron pyrite (FeS₂): Also known as fool's gold, it's a common iron sulfide mineral.
- Galena (PbS): A lead sulfide mineral, an important source of lead.
- Sphalerite (ZnS): A zinc sulfide mineral, a principal ore of zinc.
- Cinnabar (HgS): A mercury sulfide mineral, historically a primary source of mercury.
These minerals often form the basis for extracting valuable metals. Their formation relies on the inherent ability of sulfur to form stable anionic bonds with various metals.
2. Hydrogen Sulfide (H₂S): A Pungent Gas
Hydrogen sulfide (H₂S) is a colorless gas with a characteristic rotten egg odor. While toxic at high concentrations, it plays a role in certain biological processes and industrial applications. In H₂S, sulfur isn't explicitly an ion, but it exhibits a partial negative charge due to the higher electronegativity of sulfur compared to hydrogen. This partial negative charge highlights sulfur's propensity to attract electrons.
3. Sulfates (SO₄²⁻): Crucial in Biology and Industry
Sulfate (SO₄²⁻) is a polyatomic anion containing sulfur bonded to four oxygen atoms. It's a vital component in various biological and industrial processes:
- Biological Significance: Sulfates are essential for the synthesis of certain amino acids, playing a role in protein formation.
- Industrial Applications: Sulfates are found in various industrial chemicals, including sulfuric acid, a cornerstone of many industrial processes.
The sulfate anion's stability highlights sulfur's versatility in forming complex anions with other elements.
Rare Instances of Sulfur in Cationic Form
While the formation of the sulfide anion (S²⁻) is the dominant behavior of sulfur, there are extremely rare and highly specialized circumstances where sulfur can exhibit cationic behavior. These are generally under very specific, highly energetic conditions and involve highly unusual bonding. They are not typical behavior and are exceptions rather than the rule.
High Oxidation States and Unusual Bonding Environments
In highly oxidizing environments, and with highly electronegative elements, sulfur can theoretically exist in exceptionally high oxidation states. These states can result in the formation of species where sulfur exhibits a positive charge. However, these cases are uncommon, and the positive charge on the sulfur is often delocalized or shared across multiple atoms. They are generally not simple, single-atom cations like those formed by metals.
The Exception Proves the Rule
The existence of these rare cationic forms of sulfur doesn't negate the overwhelming evidence supporting sulfur's predominant role as an anion. These exceptional cases emphasize the complexity of chemical behavior and the influence of specific reaction conditions, but they do not change the fundamental nature of sulfur's tendency to gain electrons.
Conclusion: Sulfur's Predominantly Anionic Nature
In summary, while sulfur can exhibit unusual cationic behavior in rare and specific chemical environments, its predominant and most stable ionic form is the sulfide anion (S²⁻). This is driven by its electron configuration and the strong tendency to gain two electrons to achieve a stable octet. The formation of the sulfide ion is fundamental to understanding the behavior of sulfur in a vast array of compounds and reactions, from the formation of essential minerals to biological processes and industrial applications. Its anionic nature defines its chemical characteristics and plays a crucial role in various scientific and technological fields. Understanding this fundamental property is key to unlocking a deeper understanding of sulfur’s importance in the world around us.
Latest Posts
Latest Posts
-
What Is 20 Percent Of 200
Mar 14, 2025
-
How Many Cups In 32 Fl Oz
Mar 14, 2025
-
What Is 18 Cm In Inches
Mar 14, 2025
-
How Tall Is 80 Inches In Feet
Mar 14, 2025
-
1000 Mg Is How Many Grams
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Is Sulfur A Cation Or Anion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
