Is Supporting Combustion A Physical Property
Kalali
Mar 19, 2025 · 5 min read
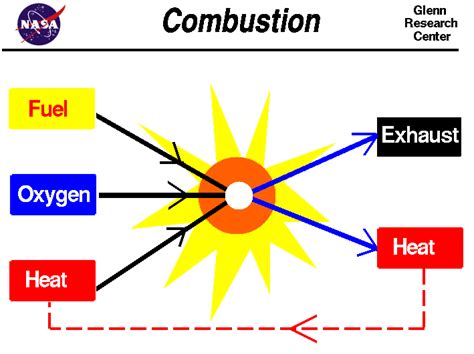
Table of Contents
Is Supporting Combustion a Physical Property? Delving into the Nature of Chemical and Physical Changes
The question of whether supporting combustion is a physical property often sparks debate. While seemingly straightforward, the answer requires a nuanced understanding of the differences between physical and chemical properties, and the intricate processes involved in combustion. This article will explore this topic in depth, examining the characteristics of combustion, the definitions of physical and chemical properties, and finally, definitively answering the question.
Understanding Combustion: A Chemical Process at its Core
Combustion, at its heart, is a chemical process. It's a rapid, exothermic (heat-releasing) redox reaction between a fuel and an oxidant, usually oxygen. This reaction results in the formation of new substances, fundamentally altering the chemical composition of the reactants. The products of combustion are often oxides, such as carbon dioxide (CO₂) and water (H₂O), when the fuel is a hydrocarbon.
Key characteristics of combustion:
- Chemical Change: The most crucial aspect is the transformation of the starting materials (fuel and oxidant) into entirely new substances. This is unlike physical changes, where the substance's chemical composition remains the same.
- Exothermic Reaction: Combustion releases a significant amount of energy in the form of heat and light. This energy release is a direct consequence of the breaking and forming of chemical bonds during the reaction.
- Rapid Oxidation: The reaction involves rapid oxidation, meaning the fuel combines with oxygen at a high rate. This rapid combination is what makes combustion appear so energetic.
- Flame Production: Often, but not always, combustion is accompanied by the production of a flame, a visible manifestation of the intense heat and chemical reactions occurring.
Examples of Combustion: Illustrating the Chemical Transformation
Let's examine a few examples to further illustrate the chemical nature of combustion:
- Burning Wood: When wood burns, the cellulose and lignin (complex organic molecules) in the wood react with oxygen. The products are ash (containing various inorganic compounds), carbon dioxide, water vapor, and other gases. The original wood is completely transformed into different substances.
- Burning Methane: Methane (CH₄), a major component of natural gas, combusts in the presence of oxygen to produce carbon dioxide and water. This chemical reaction involves the breaking of the carbon-hydrogen bonds in methane and the formation of new bonds in carbon dioxide and water.
- Burning Magnesium: Magnesium (Mg) reacts vigorously with oxygen (O₂) to produce magnesium oxide (MgO). This is a bright, exothermic reaction resulting in a white powder – a completely new substance unlike the original magnesium metal.
Defining Physical and Chemical Properties: A Critical Distinction
Before we can definitively classify whether supporting combustion is a physical or chemical property, we must clearly define each term.
Physical Properties: These are characteristics that can be observed or measured without changing the substance's chemical composition. Examples include:
- Color: The visual appearance of a substance.
- Density: Mass per unit volume.
- Melting Point: The temperature at which a solid turns into a liquid.
- Boiling Point: The temperature at which a liquid turns into a gas.
- Solubility: The ability of a substance to dissolve in a solvent.
- Conductivity: The ability of a substance to conduct heat or electricity.
Crucially, no new substances are formed when observing or measuring physical properties.
Chemical Properties: These describe how a substance reacts with other substances or its tendency to undergo chemical changes. Examples include:
- Flammability: The ability of a substance to burn in the presence of oxygen.
- Reactivity with Acids: How a substance reacts when exposed to acids.
- Toxicity: The harmful effects of a substance on living organisms.
- Corrosion Resistance: The ability of a substance to resist chemical degradation.
The key difference is that chemical properties involve a change in chemical composition, forming new substances with different properties.
Why Supporting Combustion is NOT a Physical Property
Given these definitions, it becomes clear that supporting combustion is a chemical property, not a physical property. A substance that "supports combustion," such as oxygen, actively participates in the chemical reaction of combustion. It is not merely present; it is a reactant that undergoes a chemical transformation.
Oxygen does not simply facilitate the burning process; it chemically combines with the fuel, forming new chemical bonds and resulting in new substances (the products of combustion). Observing a substance's ability to support combustion inherently involves observing its participation in a chemical reaction and, thus, a chemical change.
The Role of Oxygen: A Deeper Look into the Chemical Reaction
Oxygen's role in combustion is far more profound than simply being a passive observer. It acts as the oxidant, accepting electrons from the fuel in a redox reaction. This electron transfer is the fundamental chemical event that drives the combustion process. The oxygen atoms become incorporated into the products, permanently altering the chemical composition of both the fuel and the oxygen itself.
Consider again the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
This equation vividly demonstrates the chemical transformation. Methane and oxygen react to form entirely new molecules, carbon dioxide and water. The oxygen is not merely present; it is consumed and chemically transformed into a new substance.
Further Clarification: Addressing Potential Misconceptions
Some might argue that the physical properties of a substance, such as its state (solid, liquid, gas) or its surface area, can influence its ability to support combustion. While this is true to some extent, these factors affect the rate of combustion, not the fundamental chemical nature of the process.
A larger surface area increases the contact between fuel and oxygen, speeding up the reaction. However, this is an influence on reaction kinetics, not a redefinition of the chemical nature of combustion itself. The underlying chemical transformation remains the same.
Conclusion: A Definitive Answer
In conclusion, supporting combustion is unequivocally a chemical property. The ability of a substance to support combustion arises from its participation as a reactant in a chemical reaction, involving the breaking and forming of chemical bonds and the creation of new substances. Observing this ability intrinsically involves observing a chemical change. While physical factors can influence the rate of combustion, they do not alter the fact that supporting combustion fundamentally involves a chemical transformation. Therefore, classifying it as a physical property would be a significant mischaracterization of the chemical processes involved.
Latest Posts
Latest Posts
-
26 Out Of 32 As A Percentage
Mar 19, 2025
-
What Is 17 25 As A Percent
Mar 19, 2025
-
How Many Ml In 0 5 Oz
Mar 19, 2025
-
Como Sacar El 10 Por Ciento De Una Cantidad
Mar 19, 2025
-
1 Out Of 13 As A Percentage
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Supporting Combustion A Physical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
