Keq Is Equal To Delta G
Kalali
Apr 01, 2025 · 5 min read
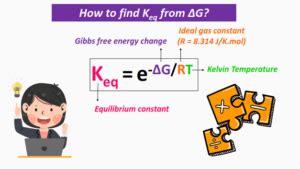
Table of Contents
Keq is Equal to ΔG: Understanding the Relationship Between Equilibrium Constant and Gibbs Free Energy
The relationship between the equilibrium constant (Keq) and the Gibbs Free Energy change (ΔG) is a cornerstone of physical chemistry, providing a powerful link between thermodynamics and kinetics. Understanding this relationship allows us to predict the spontaneity and extent of a chemical reaction. This article will delve deep into the connection between Keq and ΔG, exploring the underlying principles and providing practical examples to solidify your understanding.
The Equilibrium Constant (Keq)
The equilibrium constant, Keq, quantifies the relative amounts of reactants and products present at equilibrium for a reversible reaction. It's a ratio of the activities of products to the activities of reactants, each raised to the power of its stoichiometric coefficient. For a general reaction:
aA + bB ⇌ cC + dD
The equilibrium constant is expressed as:
Keq = ([C]^c [D]^d) / ([A]^a [B]^b)
where [A], [B], [C], and [D] represent the equilibrium concentrations (or activities) of the respective species. A large Keq value (Keq >> 1) indicates that the equilibrium lies far to the right, favoring product formation. Conversely, a small Keq value (Keq << 1) signifies that the equilibrium favors reactants. A Keq value of 1 suggests approximately equal concentrations of reactants and products at equilibrium.
Important Note: The equilibrium constant is temperature-dependent. A change in temperature will alter the value of Keq.
Factors Affecting Keq
Several factors influence the equilibrium constant:
-
Temperature: As mentioned, temperature significantly impacts Keq. For exothermic reactions (ΔH < 0), increasing the temperature decreases Keq, while for endothermic reactions (ΔH > 0), increasing the temperature increases Keq.
-
Pressure (for gaseous reactions): Changes in pressure affect the equilibrium position of gaseous reactions. Increasing the pressure favors the side with fewer gas molecules, while decreasing the pressure favors the side with more gas molecules. This is governed by Le Chatelier's principle.
-
Concentration: Changes in the concentration of reactants or products will shift the equilibrium to counteract the change, maintaining the Keq value at a constant temperature.
Gibbs Free Energy (ΔG)
Gibbs Free Energy (ΔG) is a thermodynamic potential that measures the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. It provides a criterion for the spontaneity of a process. A negative ΔG value indicates a spontaneous process (favoring product formation), while a positive ΔG value suggests a non-spontaneous process (favoring reactants). A ΔG value of zero indicates a system at equilibrium.
The Gibbs Free Energy change is related to enthalpy (ΔH), entropy (ΔS), and temperature (T) by the following equation:
ΔG = ΔH - TΔS
where:
- ΔH is the change in enthalpy (heat content)
- ΔS is the change in entropy (disorder)
- T is the absolute temperature in Kelvin
Understanding Spontaneity with ΔG
-
ΔG < 0: The reaction is spontaneous under the given conditions. Products are favored at equilibrium.
-
ΔG > 0: The reaction is non-spontaneous under the given conditions. Reactants are favored at equilibrium. The reverse reaction would be spontaneous.
-
ΔG = 0: The reaction is at equilibrium. There is no net change in the concentrations of reactants and products.
The Relationship Between Keq and ΔG
The crucial link between Keq and ΔG is expressed through the following equation:
ΔG° = -RTlnKeq
where:
- ΔG° is the standard Gibbs Free Energy change (at standard conditions: 298 K and 1 atm pressure).
- R is the ideal gas constant (8.314 J/mol·K)
- T is the absolute temperature in Kelvin
- ln represents the natural logarithm
This equation reveals that:
-
A large Keq value (Keq >> 1) corresponds to a highly negative ΔG°, indicating a spontaneous reaction strongly favoring product formation.
-
A small Keq value (Keq << 1) corresponds to a highly positive ΔG°, indicating a non-spontaneous reaction strongly favoring reactants.
-
A Keq value of 1 corresponds to a ΔG° of 0, indicating a reaction at equilibrium.
Standard vs. Non-Standard Conditions
The equation ΔG° = -RTlnKeq applies specifically to standard conditions. Under non-standard conditions, the Gibbs Free Energy change is calculated using:
ΔG = ΔG° + RTlnQ
where Q is the reaction quotient, which has the same form as Keq but uses the concentrations at any given point in the reaction, not just at equilibrium. At equilibrium, Q = Keq, and ΔG = 0.
Practical Applications and Examples
The Keq-ΔG relationship is essential in various fields:
-
Predicting reaction spontaneity: By calculating ΔG, we can predict whether a reaction will proceed spontaneously under specific conditions.
-
Determining equilibrium concentrations: Knowing Keq allows us to calculate the equilibrium concentrations of reactants and products.
-
Designing chemical processes: Understanding the equilibrium constant and Gibbs free energy helps in optimizing reaction conditions to maximize product yield.
-
Biochemistry and Enzymology: The principles are crucial for understanding metabolic pathways and enzyme-catalyzed reactions.
Example:
Consider the reaction: N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
Suppose at a certain temperature, Keq = 10^5. Using the equation ΔG° = -RTlnKeq, we can calculate ΔG°:
ΔG° = -(8.314 J/mol·K)(298 K)ln(10^5) ≈ -28.5 kJ/mol
The negative value of ΔG° indicates that the formation of ammonia from nitrogen and hydrogen is spontaneous under standard conditions.
Conclusion
The relationship between Keq and ΔG is a fundamental concept in chemistry that bridges the gap between thermodynamics and kinetics. Understanding this relationship is crucial for predicting reaction spontaneity, determining equilibrium concentrations, and optimizing chemical processes across numerous scientific and engineering disciplines. The ability to calculate and interpret both Keq and ΔG allows for a comprehensive understanding of chemical equilibrium and reaction behavior. Furthermore, the ability to apply this knowledge under both standard and non-standard conditions makes it a powerful tool for problem-solving and predictive analysis in various fields. By mastering these concepts, you can gain a profound insight into the driving forces behind chemical reactions and their equilibrium states.
Latest Posts
Latest Posts
-
How Many Centimeters Is 23 Inches
Apr 02, 2025
-
How Many Gallons Is 10 Cups
Apr 02, 2025
-
What Is 62 Degrees Celsius In Fahrenheit
Apr 02, 2025
-
Is Usa In The Northern Hemisphere
Apr 02, 2025
-
How Many Glasses Is 32 Oz Of Water
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Keq Is Equal To Delta G . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
