Natural Gas Is Heavier Than Air
Kalali
Mar 10, 2025 · 5 min read
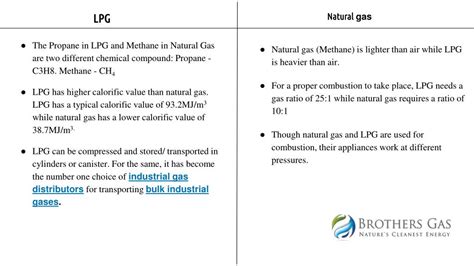
Table of Contents
Natural Gas is Heavier Than Air: Separating Fact from Fiction
The statement "natural gas is heavier than air" is a common misconception. In reality, natural gas is significantly lighter than air. This fundamental difference in density has crucial implications for safety, storage, and transportation of this vital energy source. Understanding the true nature of natural gas's density is critical for preventing accidents and ensuring responsible handling. This article will delve into the science behind the density of natural gas, explore the reasons behind the misconception, and discuss the practical consequences of this vital characteristic.
Understanding Density: A Foundation for Understanding Natural Gas
Before delving into the specifics of natural gas, let's establish a clear understanding of density. Density is simply the mass of a substance per unit volume. It's expressed in units like kilograms per cubic meter (kg/m³) or pounds per cubic foot (lb/ft³). A substance with a higher density packs more mass into a given volume than a substance with lower density. For example, lead is much denser than wood, meaning a given volume of lead weighs considerably more than the same volume of wood.
Air itself is a mixture of gases, primarily nitrogen (approximately 78%) and oxygen (approximately 21%), with trace amounts of other gases. The average density of air at sea level and standard temperature is approximately 1.225 kg/m³. This value can vary slightly depending on temperature, pressure, and humidity.
The Composition of Natural Gas: A Key Determinant of Density
Natural gas is primarily composed of methane (CH₄), a hydrocarbon with a molecular weight of 16 g/mol. Other components may include ethane, propane, butane, and various inert gases like nitrogen and carbon dioxide, but methane typically constitutes the largest percentage. The presence of these other components can slightly influence the overall density of the natural gas mixture, but the dominant influence remains the methane.
The molecular weight of methane is significantly lower than the average molecular weight of air. This directly translates to a lower density. Because methane molecules are lighter than the average air molecule, a given volume of natural gas will weigh less than the same volume of air.
Calculating the Density of Natural Gas: A Quantitative Approach
While precise calculations require knowing the exact composition of the natural gas sample, we can approximate the density using the ideal gas law. The ideal gas law states:
PV = nRT
Where:
- P = Pressure
- V = Volume
- n = Number of moles
- R = Ideal gas constant
- T = Temperature
By rearranging this equation and using the molar mass of methane (16 g/mol), we can calculate the density of pure methane. Even with the presence of other gases, the overall density will remain significantly lower than that of air. This calculation confirms the lighter-than-air nature of natural gas.
Why the Misconception? Understanding the Sources of Confusion
The misconception that natural gas is heavier than air likely stems from several factors:
-
Visual Observations: When natural gas leaks, it doesn't always rise immediately and visibly. Factors like wind, confinement in enclosed spaces, and the presence of heavier gases can influence its dispersion, creating the illusion of heavier-than-air behavior.
-
Conflation with other gases: Some natural gas components, particularly heavier hydrocarbons like propane and butane, are indeed denser than air. This leads to confusion, especially if the gas mixture is significantly enriched in these heavier components. However, pure methane and typical natural gas mixtures are lighter than air.
-
Lack of scientific understanding: A lack of basic understanding about gas density and the behavior of gases can contribute to this misconception.
-
Sensationalism and inaccurate reporting: Inaccurate or sensationalized reporting of gas leaks might inadvertently perpetuate the misconception without proper scientific clarification.
The Practical Implications of Natural Gas's Density: Safety and Handling
Understanding the true density of natural gas is crucial for several reasons:
-
Leak detection and prevention: Knowing that natural gas is lighter than air dictates where to look for leaks. Leaks will typically be found at higher elevations within buildings or near the top of storage tanks.
-
Ventilation strategies: Proper ventilation in areas where natural gas is used or stored is critical to prevent accumulation and the risk of explosions. Ventilation systems need to be designed to allow the lighter gas to escape upwards.
-
Emergency response: First responders need to understand that natural gas will tend to accumulate in upper spaces, influencing their search and rescue strategies.
-
Transportation and storage: Pipelines and storage facilities are designed to account for the buoyancy of the gas, ensuring safe and efficient transportation and storage.
-
Environmental impact: When released into the atmosphere, natural gas will tend to dissipate upwards. This consideration is important when evaluating the environmental impacts of leaks and emissions.
Debunking the Myth: Scientific Evidence and Real-World Examples
Numerous scientific studies and real-world observations confirm the lighter-than-air nature of natural gas. Laboratory experiments, field measurements, and accident investigations consistently demonstrate that methane and typical natural gas mixtures have a lower density than air. The behavior of natural gas in various scenarios, from leaks in buildings to industrial accidents, aligns with its lower density, reinforcing the accuracy of this scientific finding. Any instances where natural gas seems to behave differently are often attributable to specific environmental conditions or the presence of heavier gas components.
Conclusion: Accuracy and Safety in Handling Natural Gas
The assertion that natural gas is heavier than air is fundamentally incorrect. Natural gas, primarily methane, is significantly lighter than air, a crucial fact with direct implications for its safe handling, transportation, and storage. Understanding this density difference is critical for preventing accidents, ensuring efficient leak detection, and designing effective ventilation strategies. Continuing education and accurate reporting are vital in dispelling the misconception and fostering a safer environment around the handling of this vital energy source. The correct understanding of natural gas density is not just a scientific detail; it's a cornerstone of safe and responsible energy practices. By accurately understanding its properties, we can minimize risks and ensure the efficient and sustainable use of this valuable resource.
Latest Posts
Latest Posts
-
How Much Older Is John The Baptist Than Jesus
Jul 12, 2025
-
How Many Teaspoons In A Pound Of Sugar
Jul 12, 2025
-
How Do You Pass Level 12 On Bloxorz
Jul 12, 2025
-
How Far Is 0 4 Miles To Walk
Jul 12, 2025
-
What Is 20 Percent Of 800 000
Jul 12, 2025
Related Post
Thank you for visiting our website which covers about Natural Gas Is Heavier Than Air . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.