Neutralization Reaction Of Naoh And Hcl
Kalali
Mar 24, 2025 · 5 min read
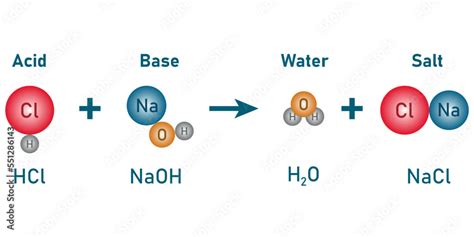
Table of Contents
Neutralization Reaction: NaOH and HCl – A Deep Dive
The neutralization reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) is a classic example of an acid-base reaction, a cornerstone concept in chemistry. Understanding this reaction provides a solid foundation for grasping broader principles of acid-base chemistry, stoichiometry, and titrations. This comprehensive article will explore this reaction in detail, covering its mechanism, applications, and practical implications.
Understanding the Reactants
Before delving into the reaction itself, let's examine the properties of the individual reactants:
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as caustic soda or lye, is a strong alkali. Its key characteristics include:
- Strong Base: It completely dissociates in water, releasing hydroxide ions (OH⁻) which readily accept protons (H⁺). This complete dissociation is crucial in understanding the reaction's completeness.
- Highly Reactive: NaOH is corrosive and reacts vigorously with acids and many other substances. Safety precautions are essential when handling it.
- Versatile Applications: It's used extensively in various industries, including soap making, paper production, and water treatment.
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid, meaning it also fully dissociates in water. Its properties include:
- Strong Acid: It readily donates protons (H⁺) to bases. This ability to donate protons is the defining characteristic of an acid.
- Highly Corrosive: Similar to NaOH, HCl is corrosive and requires careful handling.
- Industrial Importance: HCl is vital in various industrial processes, including the production of chemicals, metal cleaning, and food processing.
The Neutralization Reaction: NaOH + HCl
The neutralization reaction between NaOH and HCl is represented by the following balanced chemical equation:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This equation illustrates the reaction's essence:
- Reactants: NaOH (sodium hydroxide) and HCl (hydrochloric acid) in aqueous (aq) solution.
- Products: NaCl (sodium chloride, common table salt) also in aqueous solution and H₂O (water) in liquid (l) form.
Mechanism: The reaction occurs through a proton transfer from the acid (HCl) to the base (NaOH). The hydrogen ion (H⁺) from the HCl combines with the hydroxide ion (OH⁻) from the NaOH to form a water molecule (H₂O). The remaining sodium ion (Na⁺) and chloride ion (Cl⁻) remain in solution, forming an aqueous solution of sodium chloride.
Complete vs. Partial Neutralization
The term "neutralization" implies a complete reaction, where all the acid and base are consumed, leaving only the salt and water. However, in practice, complete neutralization might not always be achieved. This depends on factors like:
- Stoichiometry: The molar ratio of acid to base. If the ratio isn't 1:1 (as in the balanced equation), one reactant will be in excess, resulting in a non-neutral solution.
- Strength of Acid and Base: While both NaOH and HCl are strong, if a weak acid or base were involved, the neutralization would be less complete.
Determining the Endpoint: Titration
To accurately determine when complete neutralization occurs, a process called titration is used. This involves gradually adding a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until the reaction is complete. The endpoint of the titration, indicating complete neutralization, is often determined using an indicator that changes color at the neutral pH (approximately 7). In the NaOH and HCl reaction, a common indicator is phenolphthalein, which is colorless in acidic solutions and pink in basic solutions. The endpoint is reached when a single drop of titrant causes a permanent color change.
Applications of the NaOH and HCl Neutralization Reaction
The neutralization reaction between NaOH and HCl has numerous applications across various fields:
1. Industrial Chemistry:
- Wastewater Treatment: Neutralization is crucial for treating industrial wastewater containing either acidic or basic components. Adding NaOH to acidic waste and vice versa ensures environmentally safe disposal.
- Chemical Synthesis: Controlled neutralization reactions are essential in the synthesis of many chemicals, ensuring specific pH conditions are maintained.
2. Analytical Chemistry:
- Titration Analysis: As discussed earlier, titrations using NaOH and HCl are fundamental for determining the concentration of unknown solutions. This technique finds applications in various analytical settings, including quality control and environmental monitoring.
3. Food and Beverage Industry:
- pH Adjustment: Neutralization helps maintain the desired pH levels in food and beverage processing, affecting taste, texture, and preservation.
4. Everyday Applications:
- Antacid Action: While not directly involving HCl and NaOH, the principle of neutralization is fundamental to how antacids work. Antacids contain bases that neutralize excess stomach acid (HCl).
Safety Precautions
It's crucial to emphasize the importance of safety when handling NaOH and HCl. Both are corrosive chemicals that can cause severe burns and injuries. Always:
- Wear appropriate personal protective equipment (PPE): This includes gloves, eye protection, and a lab coat.
- Work in a well-ventilated area: The fumes can be irritating.
- Handle carefully: Avoid spills and direct contact with skin and eyes.
- Dispose of chemicals properly: Follow local regulations for disposal of chemical waste.
Beyond the Basics: Exploring Further
The neutralization reaction between NaOH and HCl provides a solid foundation for understanding more complex acid-base reactions. Further exploration could include:
- Weak Acid-Strong Base Neutralization: Investigating reactions involving weak acids (e.g., acetic acid) and strong bases would demonstrate the differences in completeness compared to the strong acid-strong base reaction.
- Titration Curves: Graphically representing pH changes during a titration provides valuable insights into the reaction's progress and the equivalence point.
- Thermochemistry of Neutralization: Examining the heat changes associated with the reaction provides insights into the energy involved.
- Salt Hydrolysis: Exploring the behavior of the salt formed (NaCl in this case) in solution and its potential impact on pH.
Conclusion
The neutralization reaction of NaOH and HCl is a fundamental concept in chemistry with broad applications in diverse fields. Understanding the reaction's mechanism, stoichiometry, and practical implications is crucial for various scientific and industrial processes. By understanding this seemingly simple reaction, we gain a deeper appreciation for the elegance and importance of acid-base chemistry. Remember to always prioritize safety when working with these chemicals. The knowledge gained from studying this reaction forms a strong basis for further exploration into more complex chemical phenomena.
Latest Posts
Latest Posts
-
16 Cups Is How Many Ounces
Mar 26, 2025
-
How Much Is 60 Inches In Centimeters
Mar 26, 2025
-
Cuanto Es 67 Grados Fahrenheit En Centigrados
Mar 26, 2025
-
How Many 24 Cm In Inches
Mar 26, 2025
-
67 In Is How Many Feet
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Neutralization Reaction Of Naoh And Hcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
