Noble Gas Below Krypton On The Periodic Table
Kalali
Mar 13, 2025 · 6 min read
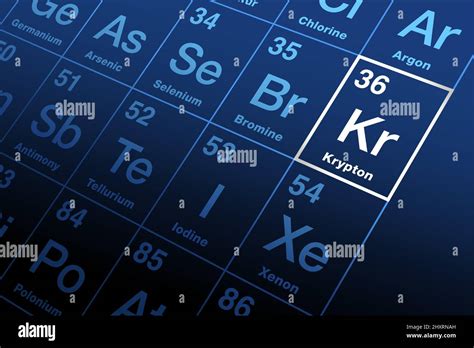
Table of Contents
Noble Gases Below Krypton: Exploring the Heavier Elements of Group 18
The noble gases, also known as inert gases, comprise Group 18 of the periodic table. These elements are renowned for their exceptional stability and minimal reactivity, a characteristic stemming from their complete valence electron shells. While helium (He), neon (Ne), and argon (Ar) are relatively common and readily studied, the noble gases below krypton (Kr)—krypton itself, xenon (Xe), radon (Rn), and the synthetic oganesson (Og)—present unique challenges and fascinating properties worthy of exploration. This article delves into the characteristics, applications, and intriguing aspects of these heavier noble gases.
Krypton: The Versatile Noble Gas
Krypton, with an atomic number of 36, occupies a pivotal position bridging the lighter, more readily available noble gases and the heavier, rarer elements. While still relatively unreactive, krypton exhibits slightly more reactivity than its lighter counterparts, opening up possibilities for compound formation under specific conditions.
Properties of Krypton:
- Abundance: Krypton is a trace element in the Earth's atmosphere, present in significantly lower concentrations than argon. Its extraction typically involves fractional distillation of liquefied air.
- Physical Properties: Krypton is a colorless, odorless, and tasteless gas under standard conditions. It exists as a monatomic gas, meaning its atoms do not naturally bond to form molecules. It has a relatively high density compared to the lighter noble gases.
- Chemical Properties: Although generally inert, krypton can form compounds under extreme conditions, primarily with highly electronegative elements like fluorine. These compounds are often unstable and require specialized conditions for their synthesis.
Applications of Krypton:
- Lighting: Krypton finds its most significant application in lighting technologies. Krypton-filled incandescent lamps produce a brighter and whiter light than those filled with argon, while krypton fluoride lasers are used in various applications including laser surgery and micromachining.
- Photography: Krypton flash lamps provide a high-intensity, short-duration light source useful in high-speed photography.
- Excimer Lasers: Krypton plays a crucial role in excimer lasers, particularly those utilizing krypton fluoride (KrF), which have applications in microlithography for semiconductor manufacturing and laser eye surgery.
Xenon: The Most Reactive Noble Gas
Xenon, with atomic number 54, marks a significant step in reactivity compared to lighter noble gases. Its larger atomic size and greater polarizability mean its outer electrons are less tightly bound, making it susceptible to chemical reactions under appropriate conditions.
Properties of Xenon:
- Abundance: Xenon is even rarer than krypton, present in trace amounts in the Earth's atmosphere. Its extraction requires specialized techniques involving cryogenic distillation.
- Physical Properties: Similar to krypton, xenon is a colorless, odorless, and tasteless gas under standard conditions. However, it possesses a higher density and boiling point than krypton.
- Chemical Properties: Xenon exhibits considerably greater reactivity than krypton. It forms a range of stable compounds with highly electronegative elements such as fluorine and oxygen, including xenon hexafluoride (XeF₆) and xenon tetroxide (XeO₄). These compounds demonstrate a fascinating exception to the long-held belief in the complete inertness of noble gases.
Applications of Xenon:
- Lighting: Xenon arc lamps produce a very intense, bright light with a color temperature close to sunlight. They are used in high-intensity discharge lighting, such as automotive headlights and studio lighting.
- Medical Imaging: Xenon is used in medical imaging techniques, particularly in nuclear medicine for brain imaging. Xenon-133, a radioactive isotope, is inhaled by patients, and its distribution throughout the brain is monitored to assess blood flow and diagnose various neurological conditions.
- Anesthesia: Xenon, while expensive, is being explored as a potential anesthetic agent due to its rapid onset and offset, and its generally benign effects on the body.
Radon: The Radioactive Noble Gas
Radon (atomic number 86) stands out among the noble gases due to its radioactivity. All naturally occurring isotopes of radon are radioactive, making it a significant concern for human health.
Properties of Radon:
- Abundance: Radon is a product of the radioactive decay of radium, uranium, and thorium present in rocks and soil. It's a gas, so it can seep into buildings from the ground.
- Physical Properties: Radon is a colorless, odorless, and tasteless gas at room temperature. It is the densest of all noble gases.
- Chemical Properties: Similar to other noble gases, radon’s reactivity is extremely low. However, its radioactivity overshadows its chemical properties, posing a health hazard.
Health Concerns and Applications of Radon:
- Health Risks: Radon is a known carcinogen, and prolonged exposure to high levels of radon in buildings can significantly increase the risk of lung cancer. Radon testing is recommended in homes to assess exposure levels and implement mitigation strategies.
- Medical Applications (Limited): Despite its toxicity, radon, in highly controlled settings, has found limited applications in radiotherapy.
Oganesson: The Synthetic Superheavy Element
Oganesson (atomic number 118) represents the heaviest noble gas and the only synthetically produced member of Group 18. Its existence challenges our understanding of the periodic table and the behavior of superheavy elements.
Properties of Oganesson:
- Abundance: Oganesson does not exist naturally. It has been synthesized in minute quantities in laboratories through nuclear reactions.
- Physical Properties: Due to its extremely short half-life, only a handful of atoms of oganesson have ever been created, making its physical properties largely unknown. Predictions based on theoretical models suggest it might be a solid at room temperature, unlike the gaseous lighter noble gases.
- Chemical Properties: Predicting oganesson's chemical properties is challenging. While expected to exhibit some noble gas characteristics, its exceptionally high atomic number might lead to unexpected behaviors, potentially deviating from the typical trends observed in lighter noble gases. Relativistic effects, influencing electron behavior at high atomic numbers, play a significant role in predicting its properties.
Challenges and Future Research in Oganesson:
Synthesizing and studying oganesson presents significant challenges due to its extremely short half-life, limited production, and intense radioactivity. Future research aims to:
- Synthesize more atoms: Increasing the production of oganesson atoms will allow for more detailed studies of its properties.
- Explore its chemical behavior: Investigating whether oganesson truly exhibits noble gas behavior or displays unexpected reactivity under specific conditions is a key research goal.
- Understanding relativistic effects: Studying oganesson’s behavior will provide valuable insights into relativistic effects in heavy elements and refine our understanding of the periodic table's extension beyond the known elements.
Conclusion: Beyond Krypton – A Realm of Intrigue
The noble gases below krypton present a fascinating realm of chemical and physical properties. While sharing the general characteristic of low reactivity, their properties vary significantly, particularly as we move towards heavier elements. Xenon's ability to form compounds shatters the long-held perception of noble gases as completely inert. Radon's radioactivity introduces a critical health and safety dimension, while oganesson, a synthetic element, pushes the boundaries of our knowledge about superheavy elements and the periodic table itself. Continued research into these elements promises further revelations and a deeper understanding of the fundamental principles of chemistry and nuclear physics. Understanding their unique characteristics opens doors to exciting advancements in diverse fields, from lighting technology and medical imaging to our fundamental understanding of the universe.
Latest Posts
Latest Posts
-
What Has 4 Letters Sometimes Has 9
Jul 02, 2025
-
How Many Milliliters In A Half Gallon
Jul 02, 2025
-
How Much Can I Sell Bro For
Jul 02, 2025
-
How Many Bottles Is 64 Oz Of Water
Jul 02, 2025
-
How Many Ounces In Pound Of Meat
Jul 02, 2025
Related Post
Thank you for visiting our website which covers about Noble Gas Below Krypton On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.