Number Of Valence Electrons Of Potassium
Kalali
Mar 25, 2025 · 5 min read
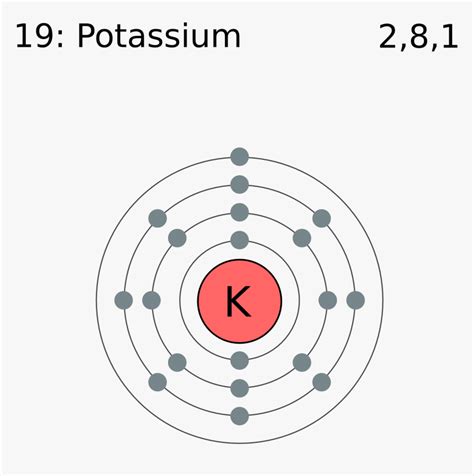
Table of Contents
The Valence Electrons of Potassium: A Deep Dive
Potassium, a crucial element for life and a common sight in chemistry labs, holds a significant place in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its reactivity and properties. This article delves into the fascinating world of potassium's valence electrons, exploring its atomic structure, chemical behavior, and applications.
Understanding Valence Electrons
Before focusing on potassium, let's establish a fundamental understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they determine an atom's chemical properties, influencing its ability to form bonds with other atoms. They participate directly in chemical reactions, determining an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). Atoms strive for stability, often achieving this by gaining, losing, or sharing valence electrons to attain a full outermost electron shell, a configuration often referred to as a noble gas configuration.
Potassium's Atomic Structure: Unveiling the Secret to its Valence Electrons
Potassium (K), atomic number 19, resides in Group 1 (also known as Alkali Metals) of the periodic table. This placement gives us immediate clues about its electronic configuration and the number of valence electrons it possesses. The atomic number, 19, indicates that a neutral potassium atom contains 19 protons and 19 electrons. These electrons are arranged in specific energy levels or shells surrounding the nucleus. Understanding this arrangement is paramount to pinpointing the valence electrons.
The electronic configuration of potassium is 1s²2s²2p⁶3s²3p⁶4s¹. This notation indicates the distribution of electrons across various energy levels:
- 1s²: Two electrons in the first energy level (closest to the nucleus).
- 2s²2p⁶: Eight electrons in the second energy level.
- 3s²3p⁶: Eight electrons in the third energy level.
- 4s¹: One electron in the fourth energy level.
The outermost shell of potassium is the fourth energy level (n=4), which contains only one electron. Therefore, potassium has one valence electron.
Visualizing Potassium's Electronic Structure
Imagine the nucleus as the sun, and the electrons orbiting around it like planets in different orbits. The first orbit (1s) is closest to the sun and holds only two "planets" (electrons). The next orbit (2s and 2p) holds eight, followed by another eight in the third orbit (3s and 3p). The fourth orbit, far from the sun, has just one solitary electron, our valence electron. This lone electron is relatively loosely held, making it easily lost in chemical reactions.
Potassium's Reactivity: The Role of its Valence Electron
The single valence electron is the primary reason for potassium's high reactivity. Potassium readily loses this electron to achieve a stable electron configuration similar to Argon (a noble gas), which has a full outermost shell. This electron loss results in the formation of a positively charged potassium ion, K⁺. This tendency to lose an electron explains potassium's characteristic behavior as an alkali metal.
Reactions with Non-Metals: Ionic Bonding
Potassium readily reacts with non-metals, such as chlorine (Cl), to form ionic compounds. Potassium loses its valence electron to chlorine, which gains that electron to fill its outermost shell. The electrostatic attraction between the resulting positively charged potassium ion (K⁺) and the negatively charged chloride ion (Cl⁻) forms an ionic bond, resulting in the formation of potassium chloride (KCl), a common salt. This type of reaction is highly exothermic, meaning it releases a considerable amount of energy.
Reactions with Water: A Vigorous Reaction
Potassium's reaction with water is particularly dramatic. The single valence electron is easily transferred to a water molecule, forming a potassium ion (K⁺) and a hydroxide ion (OH⁻). The released hydrogen atoms combine to form hydrogen gas (H₂), which is highly flammable. This reaction is highly exothermic, often leading to the ignition of the hydrogen gas, producing a characteristic lilac flame.
Applications of Potassium: Leveraging its Properties
Potassium's unique properties, largely driven by its single valence electron, lead to a wide range of applications:
Biological Significance: Essential for Life
Potassium plays a vital role in biological systems. It's an essential electrolyte, involved in maintaining fluid balance, nerve impulse transmission, and muscle contraction. Inadequate potassium levels can lead to serious health issues.
Industrial Uses: Fertilizers and Other Applications
Potassium compounds find extensive use in fertilizers, providing potassium, a crucial macronutrient for plant growth. Potassium hydroxide (KOH) is used in various industrial processes, including soap manufacturing and as a strong base in chemical reactions. Potassium permanganate (KMnO₄) is a powerful oxidizing agent with applications in water purification and as a disinfectant.
Scientific Research: Exploring Its Properties
Potassium's unique properties make it a subject of ongoing scientific research. Its reactivity is exploited in various chemical reactions, and its ionic nature makes it valuable in electrochemical studies. Isotopes of potassium are utilized in various scientific applications, including dating techniques and medical imaging.
Conclusion: Potassium's Single Valence Electron: A Key to Understanding its Behavior
The single valence electron of potassium is the key to understanding its chemical behavior and diverse applications. Its tendency to lose this electron to achieve a stable electron configuration drives its reactivity, resulting in the formation of ionic compounds and its vigorous reactions with non-metals and water. This single electron is the foundation for potassium's crucial role in biological systems and its widespread applications in industry and scientific research. The story of potassium's valence electron is a testament to the fundamental role of electronic structure in determining the properties and behavior of elements. Further exploration of this topic allows for a deeper understanding of the principles of chemistry and the remarkable diversity of the elements in the periodic table. Understanding valence electrons provides a fundamental framework for comprehending chemical reactivity and the behavior of matter, reinforcing the interconnectedness of atomic structure, chemical properties, and applications. Therefore, the seemingly simple concept of a single valence electron in potassium reveals a complex world of chemical interactions and technological applications.
Latest Posts
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons Of Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
