Orbital Blocks Of The Periodic Table
Kalali
Mar 24, 2025 · 7 min read
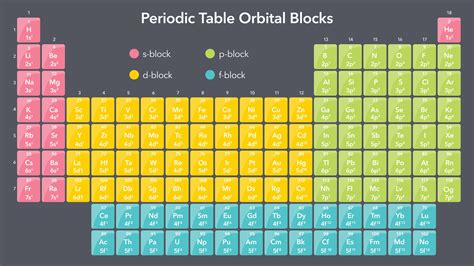
Table of Contents
Understanding the Orbital Blocks of the Periodic Table: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. While the table's arrangement might seem arbitrary at first glance, it reflects a profound underlying order governed by the filling of electron orbitals. Understanding the orbital blocks—s-block, p-block, d-block, and f-block—is crucial to grasping the periodic trends and predicting the behavior of elements. This article will delve into each block, exploring its characteristic properties, representative elements, and the underlying quantum mechanics that dictate their structure.
The Significance of Electron Orbitals
Before diving into the orbital blocks, let's briefly review the concept of electron orbitals. According to quantum mechanics, electrons within an atom don't occupy fixed positions but rather exist in regions of space called orbitals. These orbitals are described by quantum numbers (principal quantum number, n; azimuthal quantum number, l; magnetic quantum number, ml; and spin quantum number, ms), which define their energy levels, shapes, and orientations. The principal quantum number (n) dictates the energy level, and the azimuthal quantum number (l) determines the orbital's shape and type (s, p, d, f).
-
s-orbitals (l=0): These are spherical orbitals, with the electron density concentrated around the nucleus. They can accommodate a maximum of two electrons.
-
p-orbitals (l=1): These have a dumbbell shape, with three mutually perpendicular p-orbitals (px, py, pz) existing within a given energy level. Each p-orbital can hold a maximum of two electrons, for a total of six electrons per energy level.
-
d-orbitals (l=2): More complex in shape than s and p orbitals, the five d-orbitals (dxy, dyz, dxz, dx²-y², dz²) can hold a maximum of ten electrons.
-
f-orbitals (l=3): These have even more intricate shapes, with seven f-orbitals capable of holding a maximum of fourteen electrons.
Exploring the Orbital Blocks
The periodic table's organization directly mirrors the sequential filling of these electron orbitals. Each block is named after the type of orbital that is being filled with electrons as you move across the table within that block.
1. The s-Block Elements: Alkali Metals and Alkaline Earth Metals
The s-block encompasses the first two groups of the periodic table—Groups 1 (alkali metals) and 2 (alkaline earth metals). Elements in this block have their valence electrons (outermost electrons) residing in s-orbitals.
Characteristics of s-block elements:
- Low ionization energies: They readily lose their valence electrons, forming positively charged ions (cations). This explains their high reactivity, particularly in the alkali metals.
- Electropositive nature: They tend to lose electrons and form ionic compounds.
- Metallic character: All s-block elements exhibit metallic properties such as conductivity and malleability.
- Reactivity increases down the group: As you move down the group, the valence electrons are further from the nucleus, making them easier to remove. Hence, reactivity increases.
Examples of s-block elements:
- Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr): Alkali metals – highly reactive, readily react with water and air.
- Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra): Alkaline earth metals – less reactive than alkali metals but still display significant reactivity.
2. The p-Block Elements: A Diverse Group
The p-block elements occupy Groups 13 to 18 of the periodic table. Their valence electrons fill the p-orbitals. This block encompasses a wide variety of elements, showing a greater range of properties compared to the s-block.
Characteristics of p-block elements:
- Variable oxidation states: They can exhibit multiple oxidation states, often leading to a diverse range of compounds.
- Non-metallic character (mostly): While some elements display metallic properties, many are non-metals or metalloids.
- Reactivity varies greatly: Reactivity depends on factors like the number of valence electrons and electronegativity.
- Formation of covalent bonds: P-block elements frequently form covalent bonds, sharing electrons to achieve stable electron configurations.
Examples of p-block elements:
- Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl): Group 13 – possess a mixture of metallic and non-metallic properties.
- Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb): Group 14 – includes both non-metals (carbon) and metals (tin, lead).
- Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), Bismuth (Bi): Group 15 – show a gradual transition from non-metallic to metallic character.
- Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), Polonium (Po): Group 16 – important for biological processes (oxygen) and industrial applications (sulfur).
- Halogens (Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At)): Group 17 – highly reactive non-metals, readily forming anions.
- Noble gases (Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn)): Group 18 – extremely unreactive due to their full valence electron shells.
3. The d-Block Elements: Transition Metals
The d-block elements, located in the central region of the periodic table, are also known as transition metals. They are characterized by the filling of d-orbitals in their penultimate (second to outermost) electron shell.
Characteristics of d-block elements:
- Variable oxidation states: They exhibit a wide range of oxidation states, often leading to colorful compounds.
- Formation of complex ions: Transition metals readily form complex ions, involving coordination bonds with ligands.
- Catalytic activity: Many transition metals act as catalysts in various chemical reactions, often due to their variable oxidation states.
- Metallic properties: They are typically hard, strong, and have high melting and boiling points.
- Paramagnetism: Many transition metals are paramagnetic, meaning they are attracted to magnetic fields due to unpaired electrons.
Examples of d-block elements:
- Iron (Fe), Cobalt (Co), Nickel (Ni): Crucial for biological processes (hemoglobin, vitamin B12).
- Copper (Cu), Silver (Ag), Gold (Au): Excellent conductors of electricity and heat, used in various applications.
- Titanium (Ti), Chromium (Cr), Manganese (Mn): Used in alloys and as pigments.
4. The f-Block Elements: Inner Transition Metals
The f-block elements, positioned separately at the bottom of the periodic table, are also known as inner transition metals. They are divided into two series: the lanthanides (rare earth elements) and the actinides. The filling of f-orbitals characterizes these elements.
Characteristics of f-block elements:
- Similar chemical properties within a series: Elements within the same series (lanthanides or actinides) have very similar chemical properties due to the filling of the inner f-orbitals.
- Radioactivity (actinides): Most actinides are radioactive, undergoing spontaneous nuclear decay.
- Paramagnetism: Similar to d-block elements, many f-block elements display paramagnetism.
- Applications in specialized technologies: f-block elements find use in various specialized applications, including magnets, lighting, and nuclear technology.
Examples of f-block elements:
- Lanthanum (La) to Lutetium (Lu): Lanthanides – used in various applications including magnets and catalysts.
- Actinium (Ac) to Lawrencium (Lr): Actinides – most are radioactive, with some having important applications in nuclear energy.
Periodic Trends and Orbital Blocks
The orbital blocks are fundamental to understanding various periodic trends. For example:
- Atomic radius: Atomic radius generally increases down a group (due to the addition of electron shells) and decreases across a period (due to increasing nuclear charge). The d- and f-blocks show irregularities due to shielding effects and electron-electron repulsions.
- Ionization energy: Ionization energy generally decreases down a group and increases across a period. The d- and f-blocks exhibit irregularities due to factors like electron shielding and electron configuration.
- Electronegativity: Electronegativity generally decreases down a group and increases across a period. Again, the d- and f-blocks display deviations from this general trend.
Conclusion
The orbital blocks of the periodic table are not merely a convenient arrangement; they represent a deep connection between the quantum mechanical nature of atoms and the macroscopic properties of elements. By understanding the filling of s, p, d, and f orbitals, we gain a powerful tool for predicting and explaining the chemical behavior of elements. From the highly reactive alkali metals to the diverse p-block elements and the technologically important transition metals, the orbital blocks provide a framework for appreciating the richness and complexity of the periodic system, forming the very basis of chemistry itself. Further study of each block individually can reveal even more nuanced details about the chemical properties of elements, leading to a deeper understanding of the world around us.
Latest Posts
Latest Posts
-
2 Quarts Of Water In Cups
Mar 25, 2025
-
What Is 9 Out Of 12
Mar 25, 2025
-
How Much Is 100ml In Oz
Mar 25, 2025
-
2 Pounds Equals How Many Ounces
Mar 25, 2025
-
How Many Feet Is 93 In
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Orbital Blocks Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
