Oxidation Number Of S In H2so4
Kalali
Mar 29, 2025 · 5 min read
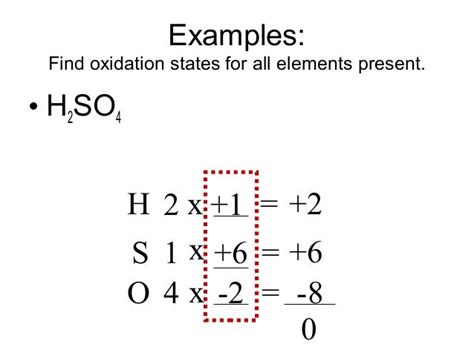
Table of Contents
Determining the Oxidation Number of Sulfur in H₂SO₄: A Comprehensive Guide
The determination of oxidation numbers is a fundamental concept in chemistry, crucial for understanding redox reactions and predicting the behavior of chemical species. This article delves deep into calculating the oxidation number of sulfur (S) in sulfuric acid (H₂SO₄), providing a step-by-step explanation, addressing common misconceptions, and exploring the broader implications of this calculation.
Understanding Oxidation Numbers
Before we dive into the specifics of H₂SO₄, let's establish a solid understanding of oxidation numbers. The oxidation number, also known as the oxidation state, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial tool for tracking electron transfer in chemical reactions. While not a true charge, it helps us predict reactivity and understand the role of different elements within a compound.
Several rules govern the assignment of oxidation numbers:
-
Rule 1: The oxidation number of an uncombined element is always zero. For example, the oxidation number of O₂ is 0, and the oxidation number of S₈ is 0.
-
Rule 2: The oxidation number of a monatomic ion is equal to its charge. For instance, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
Rule 3: The oxidation number of hydrogen is +1, except in metal hydrides where it is -1. In most compounds, hydrogen acts as a +1 cation. However, in compounds like NaH (sodium hydride), hydrogen exists as a -1 anion.
-
Rule 4: The oxidation number of oxygen is usually -2, except in peroxides (like H₂O₂) where it is -1 and in superoxides where it is -1/2. This is a very important rule, as oxygen is highly electronegative and often forms two covalent bonds, gaining two electrons.
-
Rule 5: The sum of the oxidation numbers of all atoms in a neutral molecule is zero. This is a critical rule for determining unknown oxidation numbers.
-
Rule 6: The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. This rule is similar to Rule 5 but applies to charged species.
Calculating the Oxidation Number of Sulfur in H₂SO₄
Now, let's apply these rules to determine the oxidation number of sulfur in sulfuric acid (H₂SO₄).
-
Identify the known oxidation numbers: We know from our rules that the oxidation number of hydrogen (H) is +1 and the oxidation number of oxygen (O) is -2.
-
Set up an algebraic equation: Let 'x' represent the oxidation number of sulfur (S). We have two hydrogen atoms (+1 each), one sulfur atom (x), and four oxygen atoms (-2 each). Since H₂SO₄ is a neutral molecule, the sum of the oxidation numbers must equal zero. Therefore, our equation is:
2(+1) + x + 4(-2) = 0
-
Solve for x:
2 + x - 8 = 0 x - 6 = 0 x = +6
Therefore, the oxidation number of sulfur (S) in H₂SO₄ is +6.
Why is the Oxidation Number of Sulfur +6 in H₂SO₄?
The +6 oxidation state of sulfur in H₂SO₄ reflects the high electronegativity difference between sulfur and oxygen. Oxygen atoms are highly electronegative, meaning they strongly attract electrons. In H₂SO₄, each oxygen atom essentially "pulls" electrons away from the sulfur atom, resulting in a significant positive oxidation state for sulfur. The sulfur atom shares its electrons in covalent bonds, but the unequal sharing gives sulfur a positive oxidation number.
Implications of the +6 Oxidation State of Sulfur
The +6 oxidation state of sulfur in H₂SO₄ has several important implications:
-
Strong Oxidizing Agent: Sulfuric acid is a strong oxidizing agent because the sulfur is already in a high oxidation state. It can readily accept electrons, thus oxidizing other substances. This property makes it useful in various industrial processes and chemical reactions.
-
Acidity: The high oxidation state of sulfur contributes to the strong acidity of sulfuric acid. The highly polar S=O bonds make it easy for the acid to donate protons (H⁺ ions).
-
Reactivity: The +6 oxidation state influences the reactivity of sulfuric acid with different substances. Its ability to both donate protons and accept electrons makes it a versatile reagent in many chemical reactions.
-
Stability: While sulfuric acid is a strong oxidizing agent, it's also relatively stable under normal conditions. The high oxidation state of sulfur helps to stabilize the molecule.
Common Misconceptions about Oxidation Numbers
Several common misconceptions surround oxidation numbers:
-
Oxidation numbers are not always equal to the actual charge: It's crucial to remember that oxidation numbers are hypothetical charges, not the real charges on atoms in a molecule.
-
Oxidation numbers can be fractional: In some cases, such as superoxides, oxidation numbers can be fractional.
-
Oxidation numbers are assigned based on electronegativity: The rules for assigning oxidation numbers are based on electronegativity trends, helping to determine which atom "wins" the electrons in a bond. However, directly using electronegativity to calculate an oxidation number without following the established rules can be inaccurate.
Advanced Applications and Further Exploration
The concept of oxidation numbers extends far beyond simple inorganic compounds like H₂SO₄. It's a cornerstone of:
-
Redox Reactions: Balancing redox reactions relies heavily on understanding oxidation number changes.
-
Electrochemistry: Oxidation numbers are crucial in understanding electrochemical processes and cell potentials.
-
Organic Chemistry: While less frequently used in a direct manner compared to inorganic chemistry, oxidation numbers can help understand oxidation and reduction reactions in organic compounds. For instance, identifying changes in the oxidation state of carbon atoms during oxidation reactions.
-
Inorganic Chemistry: Predicting the reactivity and stability of various inorganic compounds relies upon knowledge of oxidation states.
Conclusion
Determining the oxidation number of sulfur in H₂SO₄, which is +6, is a fundamental exercise demonstrating the application of oxidation number rules. Understanding oxidation numbers is vital in chemistry for comprehending redox reactions, predicting chemical behavior, and interpreting various chemical properties. The +6 oxidation state of sulfur significantly influences the properties of sulfuric acid, making it a strong acid and a powerful oxidizing agent. This knowledge is essential for anyone studying chemistry, whether at an introductory or advanced level. The concept extends beyond basic applications, providing a framework for comprehending complex chemical phenomena across various sub-disciplines of chemistry.
Latest Posts
Latest Posts
-
30 In Is How Many Feet
Apr 01, 2025
-
107 Cm Is How Many Inches
Apr 01, 2025
-
What Is One Percent Of A Billion
Apr 01, 2025
-
How Many Litres Is 24 Ounces
Apr 01, 2025
-
A Ray That Divides Two Congruent Angles
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of S In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
